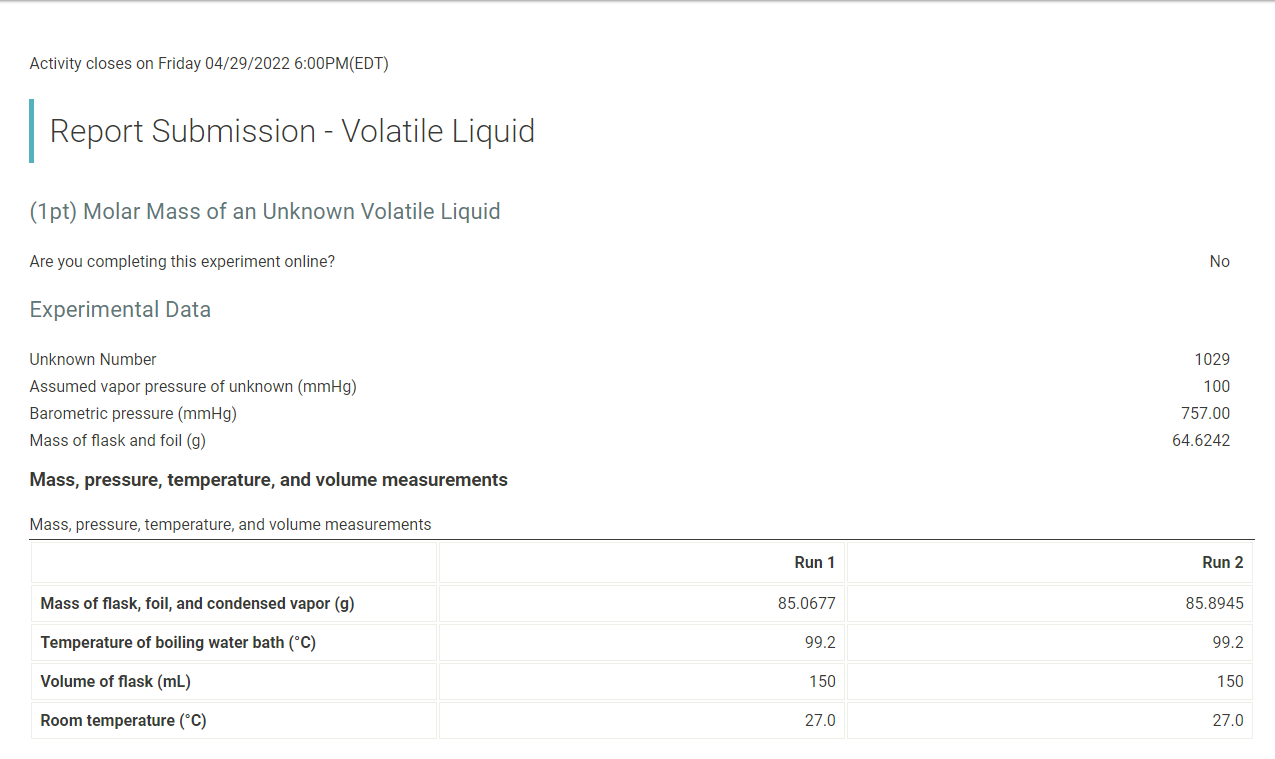
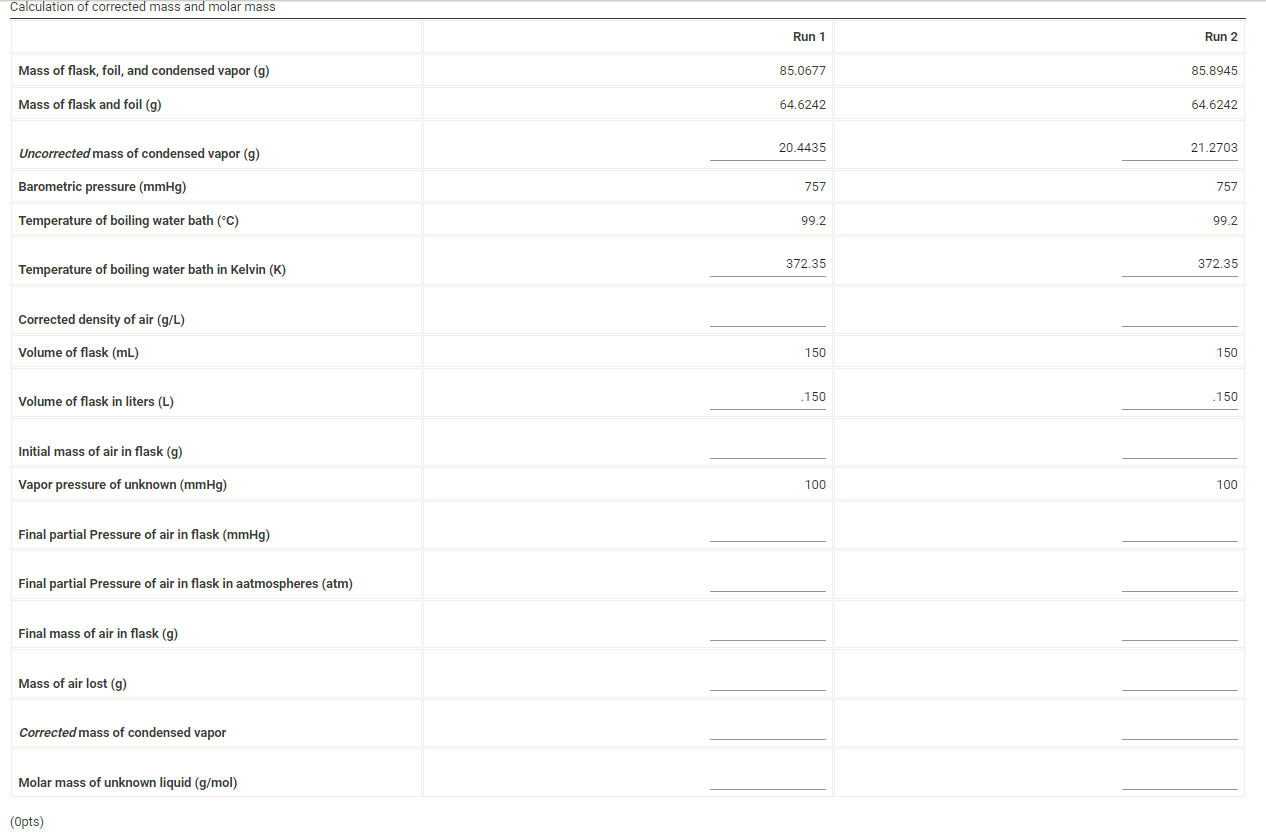
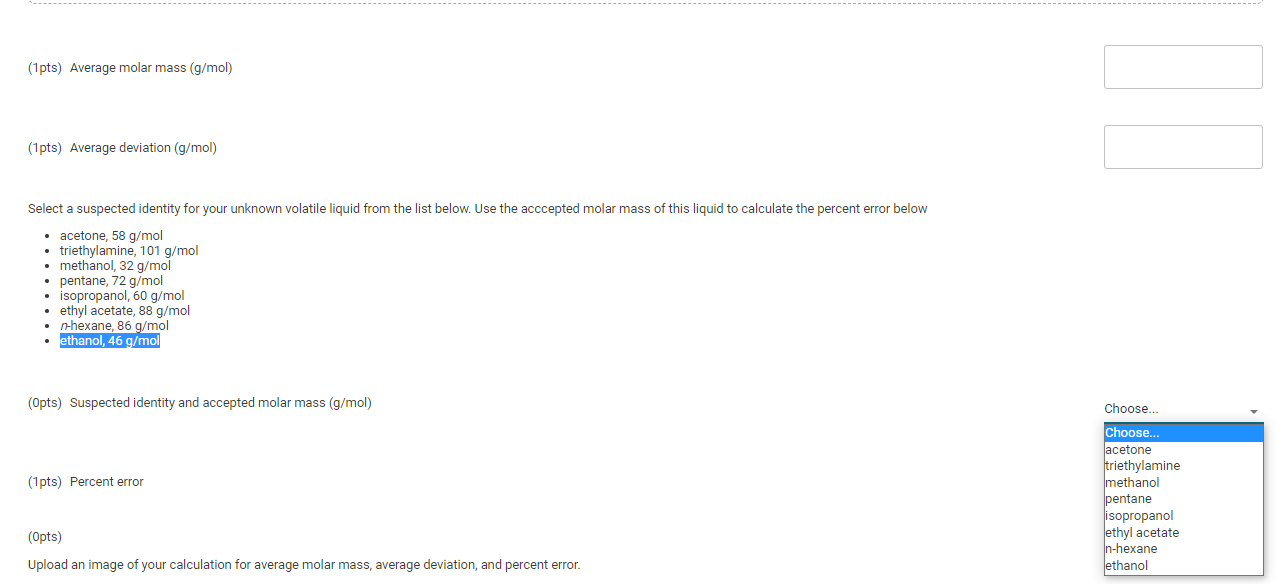

Activity closes on Friday 04/29/2022 6:00PM(EDT) | Report Submission - Volatile Liquid (1pt) Molar Mass of an Unknown Volatile Liquid Are you completing this experiment online? No Experimental Data Unknown Number Assumed vapor pressure of unknown (mmHg) Barometric pressure (mmHg) Mass of flask and foil (9) 1029 100 757.00 64.6242 Mass, pressure, temperature, and volume measurements Mass, pressure, temperature, and volume measurements Run 1 Run 2 Mass of flask, foil, and condensed vapor (9) 85.0677 85.8945 Temperature of boiling water bath (C) 99.2 99.2 Volume of flask (mL) 150 150 Room temperature (C) 27.0 27.0 Calculation of corrected mass and molar mass Run 1 Run 2 Mass of flask, foil, and condensed vapor (9) 85.0677 85.8945 Mass of flask and foil (9) 64.6242 64.6242 Uncorrected mass of condensed vapor (9) 20.4435 21.2703 Barometric pressure (mmHg) 757 757 Temperature of boiling water bath (C) 99.2 99.2 372.35 372.35 Temperature of boiling water bath in Kelvin (K) Corrected density of air (g/L) Volume of flask (mL) 150 150 Volume of flask in liters (L) .150 .150 Initial mass of air in flask (9) Vapor pressure of unknown (mmHg) 100 100 Final partial Pressure of air in flask (mmHg) Final partial Pressure of air in flask in aatmospheres (atm) Final mass of air in flask (9) Mass of air lost (9) Corrected mass of condensed vapor Molar mass of unknown liquid (g/mol) (Opts) (1pts) Average molar mass (g/mol) (1pts) Average deviation (g/mol) Select a suspected identity for your unknown volatile liquid from the list below. Use the acccepted molar mass of this liquid to calculate the percent error below acetone, 58 g/mol triethylamine, 101 g/mol methanol, 32 g/mol pentane, 72 g/mol isopropanol, 60 g/mol ethyl acetate, 88 g/mol n-hexane, 86 g/mol ethanol, 46 g/mol (Opts) Suspected identity and accepted molar mass (g/mol) (1pts) Percent error Choose... Choose... acetone triethylamine methanol pentane isopropanol ethyl acetate n-hexane ethanol (Opts) Upload an image of your calculation for average molar mass, average deviation, and percent error. (2pts) Questions 1. If the density of your unknown liquid is 0.65 g/mL, calculate the volume in liters that 3 mL of your unknown liquid would occupy when vaporized at the barometric pressure and temperature of your boiling water bath in Run 1. Use the accepted molar mass of your suspected unknown. (1pts) Volume occupied when vaporized (L) (1 pts) Based on the volume of the flask (L) from run 1, was 3 mL sufficient liquid to use in your experiment? Choose. (Opts) Choose.. yes no










