Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Activity: How many grams of iron, Fe were formed by the reaction between iron(II) oxide, Feo and carbon, C, if the reaction also yielded 22
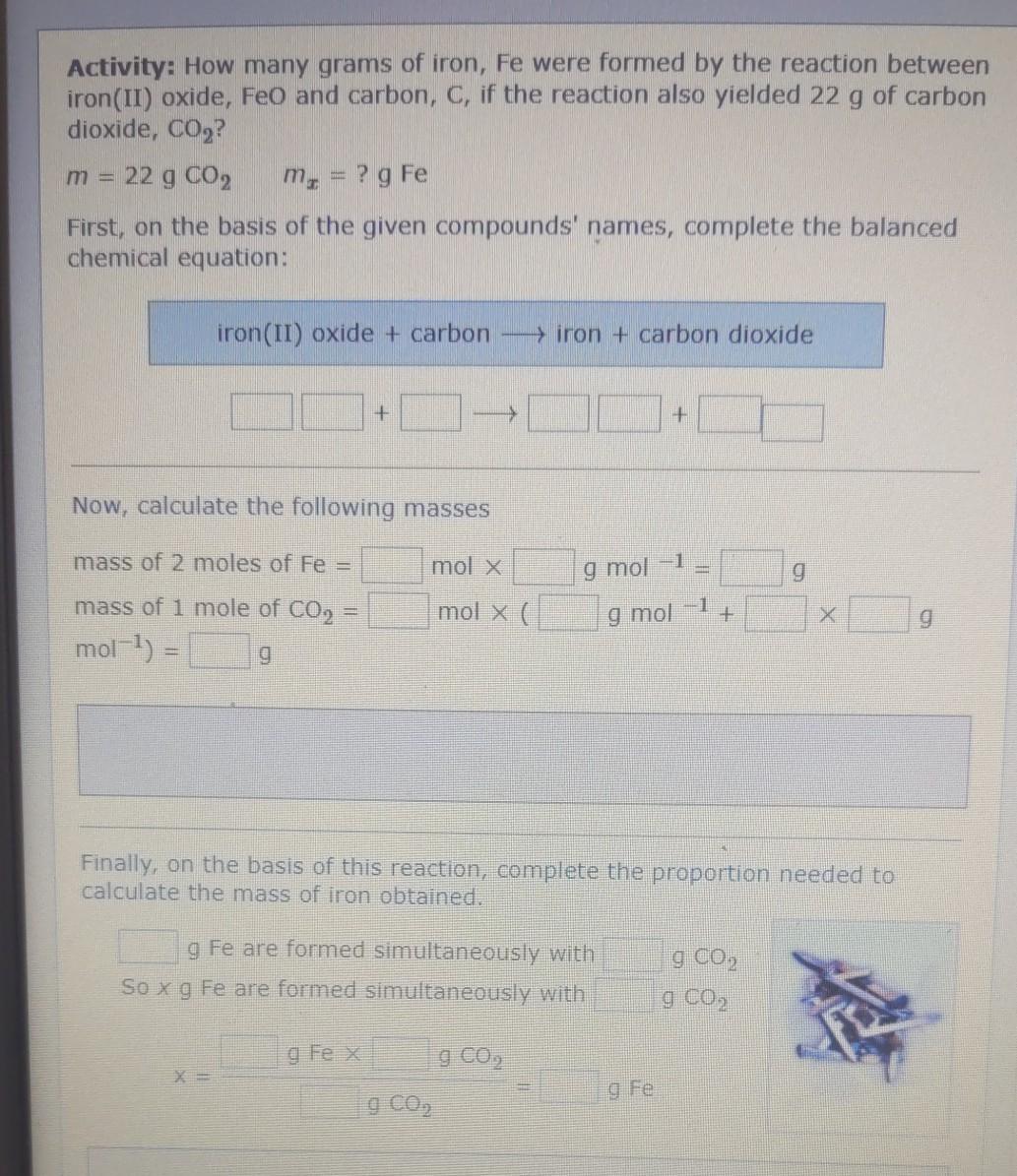
Activity: How many grams of iron, Fe were formed by the reaction between iron(II) oxide, Feo and carbon, C, if the reaction also yielded 22 g of carbon dioxide, CO2? 22 g CO2 m, = ? g Fe First, on the basis of the given compounds' names, complete the balanced chemical equation: m = iron(II) oxide + carbon > iron + carbon dioxide + + Now, calculate the following masses mass of 2 moles of Fe = mol x ] 9 mol 9 1 mol x( g mol g mass of 1 mole of CO2 = mol-1) = g Finally, on the basis of this reaction, complete the proportion needed to calculate the mass of iron obtained. g Fe are formed simultaneously with So x g Fe are formed simultaneously with g g Co, gFe x gco
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


