Question
A) Determine Activation energy using The Arrhenius Equation: k=Ae^-Ea/RT use Rate Law to help solve for k. Rate Law=k[I-][H2O2] B) Use info above to create
A) Determine Activation energy using The Arrhenius Equation: k=Ae^-Ea/RT
use Rate Law to help solve for k.
Rate Law=k[I-][H2O2]
B) Use info above to create a 6 plot include a trend line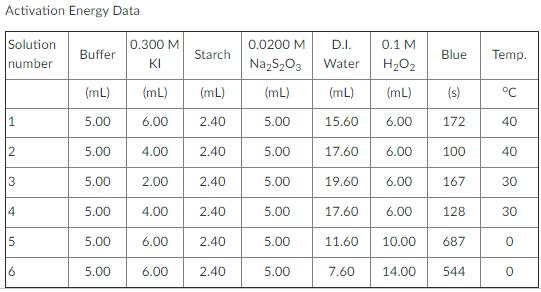
Activation Energy Data Solution 0.300 M 0.0200 M D.I. 0.1 M Buffer Starch Blue Temp. number KI NazS,03 Water H2O2 (mL) (mL) (mL) (mL) (mL) (mL) (s) C 5.00 6.00 2.40 5.00 15.60 6.00 172 40 12 5.00 4.00 2.40 5.00 17.60 6.00 100 40 3 5.00 2.00 2.40 5.00 19.60 6.00 167 30 4 5.00 4.00 2.40 5.00 17.60 6.00 128 30 5 5.00 6.00 2.40 5.00 11.60 10.00 687 5.00 6.00 2.40 5.00 7.60 14.00 544
Step by Step Solution
3.52 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



