Answered step by step
Verified Expert Solution
Question
1 Approved Answer
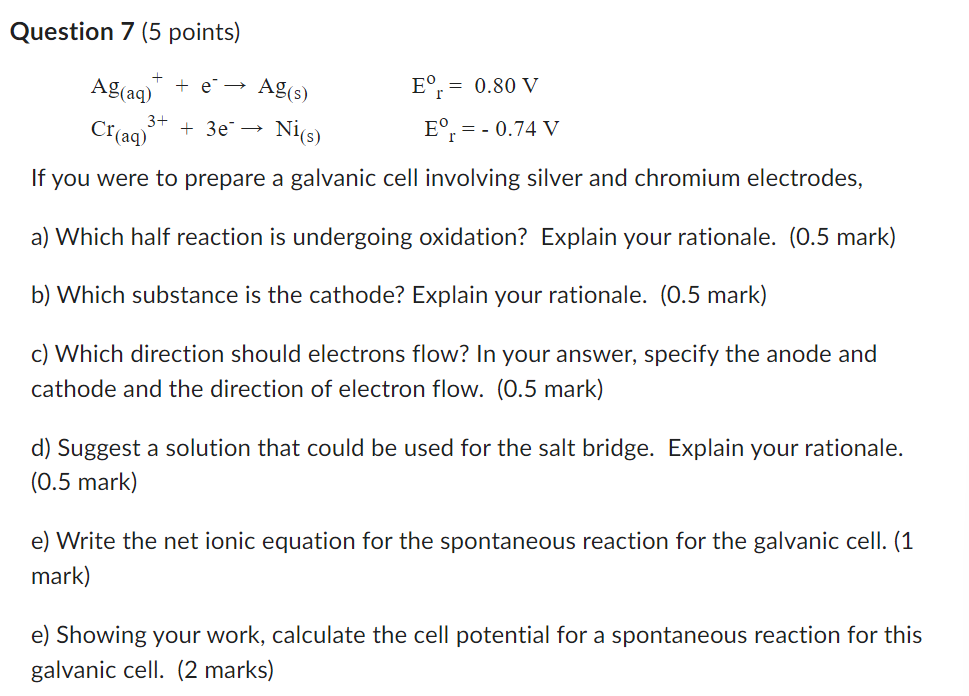
Ag ( aq ) + + e - - > Ag ( s ) Eor = 0 . 8 0 V Cr ( aq )
Agaq e Ags Eor V
Craqe Nis Eor V
If you were to prepare a galvanic cell involving silver and chromium electrodes,
a Which half reaction is undergoing oxidation? Explain your rationale. mark
b Which substance is the cathode? Explain your rationale. mark
c Which direction should electrons flow? In your answer, specify the anode and cathode and the direction of electron flow. mark
d Suggest a solution that could be used for the salt bridge. Explain your rationale. mark
e Write the net ionic equation for the spontaneous reaction for the galvanic cell. markQuestion points
If you were to prepare a galvanic cell involving silver and chromium electrodes,
a Which half reaction is undergoing oxidation? Explain your rationale. mark
b Which substance is the cathode? Explain your rationale. mark
c Which direction should electrons flow? In your answer, specify the anode and
cathode and the direction of electron flow. mark
d Suggest a solution that could be used for the salt bridge. Explain your rationale.
mark
e Write the net ionic equation for the spontaneous reaction for the galvanic cell.
mark
e Showing your work, calculate the cell potential for a spontaneous reaction for this
galvanic cell. marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


