Question
Ahmed was doing experiment with the laboratory technician and prepared standard solution in standard measuring flask by dissolving 6.5 g C3(PO4)2 in 550 ml
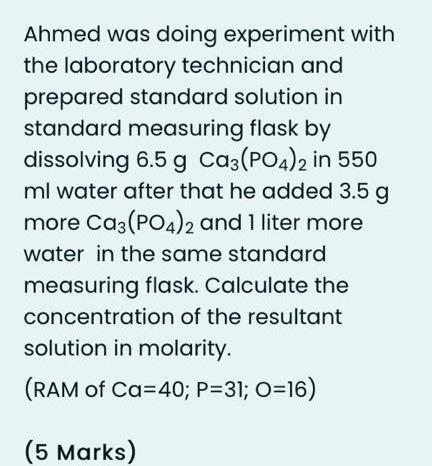
Ahmed was doing experiment with the laboratory technician and prepared standard solution in standard measuring flask by dissolving 6.5 g C3(PO4)2 in 550 ml water after that he added 3.5 g more Ca3(PO4)2 and 1 liter more water in the same standard measuring flask. Calculate the concentration of the resultant solution in molarity. (RAM of Ca=40; P=31; O=16) (5 Marks)
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the concentration of the resultant solution in molarity we need to first calculate the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



