Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Air at T = 20C and P = 100 kPa enters a compressor with a mass flow rate of m= 0.025 kg/s through a
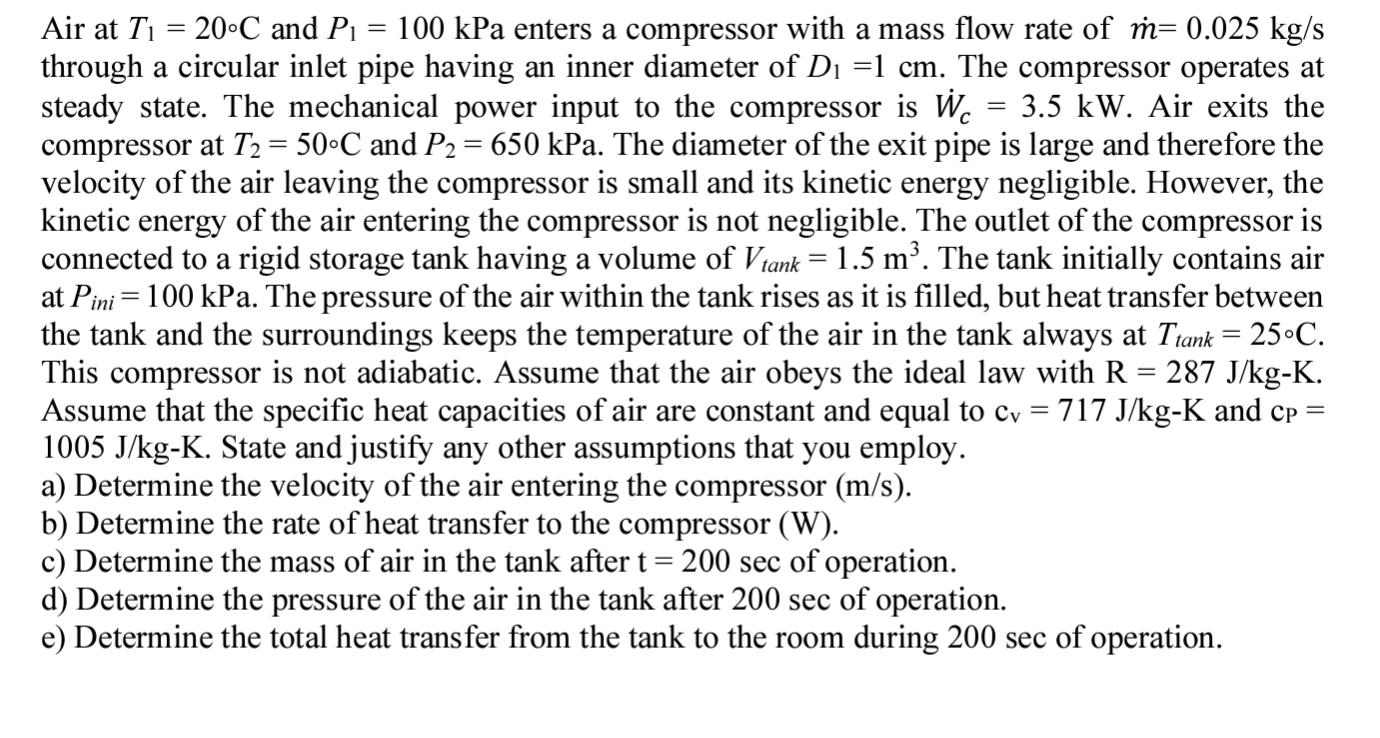
Air at T = 20C and P = 100 kPa enters a compressor with a mass flow rate of m= 0.025 kg/s through a circular inlet pipe having an inner diameter of D =1 cm. The compressor operates at steady state. The mechanical power input to the compressor is W = 3.5 kW. Air exits the compressor at T = 50C and P = 650 kPa. The diameter of the exit pipe is large and therefore the velocity of the air leaving the compressor is small and its kinetic energy negligible. However, the kinetic energy of the air entering the compressor is not negligible. The outlet of the compressor is connected to a rigid storage tank having a volume of Vtank = 1.5 m. The tank initially contains air at Pini 100 kPa. The pressure of the air within the tank rises as it is filled, but heat transfer between the tank and the surroundings keeps the temperature of the air in the tank always at Ttank = 25C. This compressor is not adiabatic. Assume that the air obeys the ideal law with R = 287 J/kg-K. Assume that the specific heat capacities of air are constant and equal to cv = 717 J/kg-K and cp = 1005 J/kg-K. State and justify any other assumptions that you employ. a) Determine the velocity of the air entering the compressor (m/s). = b) Determine the rate of heat transfer to the compressor (W). c) Determine the mass of air in the tank after t = 200 sec of operation. d) Determine the pressure of the air in the tank after 200 sec of operation. e) Determine the total heat transfer from the tank to the room during 200 sec of operation.
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
e To find the total heat t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


