Answered step by step
Verified Expert Solution
Question
1 Approved Answer
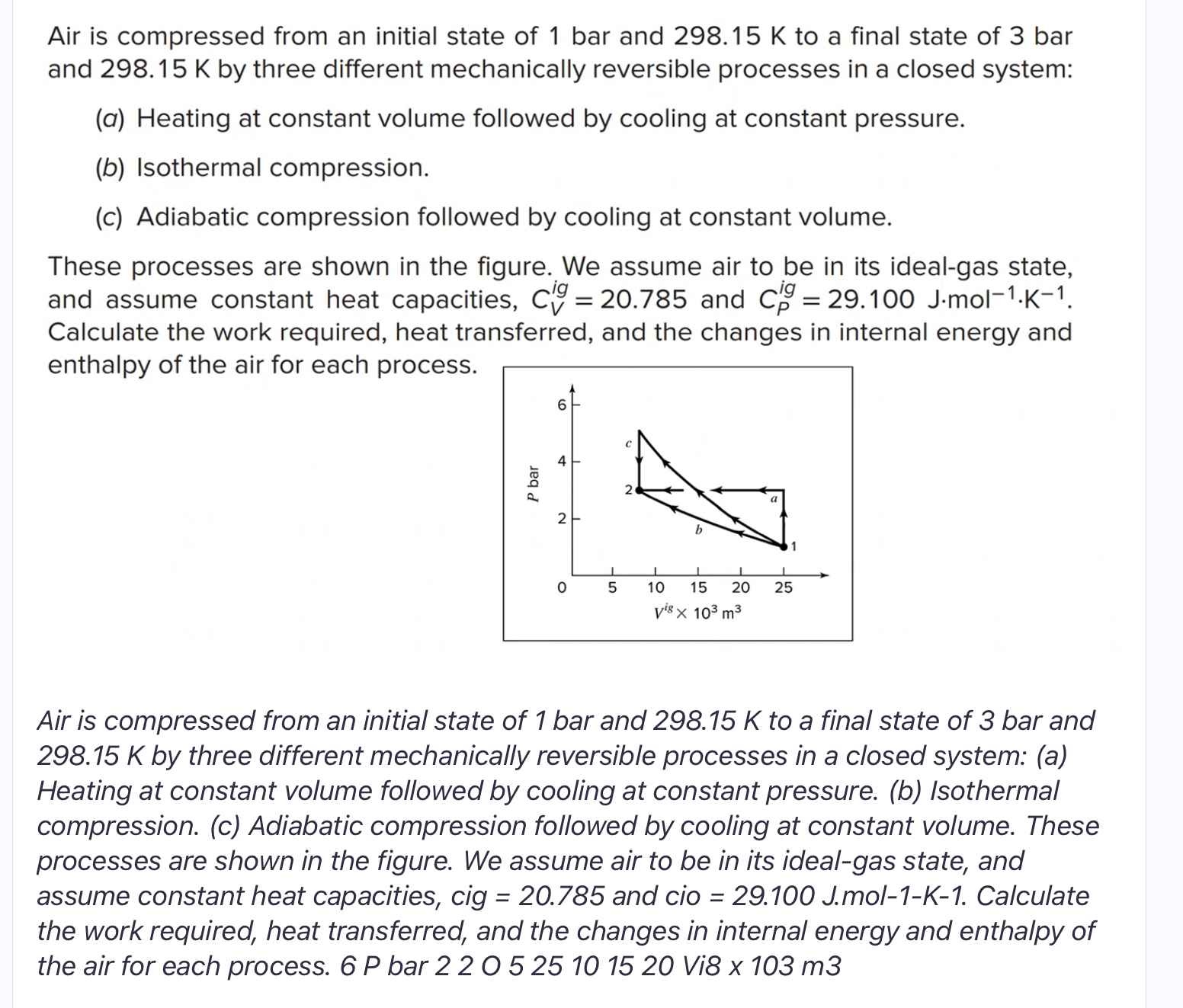
Air is compressed from an initial state of 1 bar and 2 9 8 . 1 5 K to a final state of 3 bar
Air is compressed from an initial state of bar and to a final state of bar and by three different mechanically reversible processes in a closed system:
a Heating at constant volume followed by cooling at constant pressure.
b Isothermal compression.
c Adiabatic compression followed by cooling at constant volume.
These processes are shown in the figure. We assume air to be in its idealgas state, and assume constant heat capacities, and Calculate the work required, heat transferred, and the changes in internal energy and enthalpy of the air for each process.
Air is compressed from an initial state of bar and to a final state of bar and by three different mechanically reversible processes in a closed system: a Heating at constant volume followed by cooling at constant pressure. b Isothermal compression. c Adiabatic compression followed by cooling at constant volume. These processes are shown in the figure. We assume air to be in its idealgas state, and assume constant heat capacities, cig and cio mol Calculate the work required, heat transferred, and the changes in internal energy and enthalpy of the air for each process. P bar Vi x m
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


