Answered step by step
Verified Expert Solution
Question
1 Approved Answer
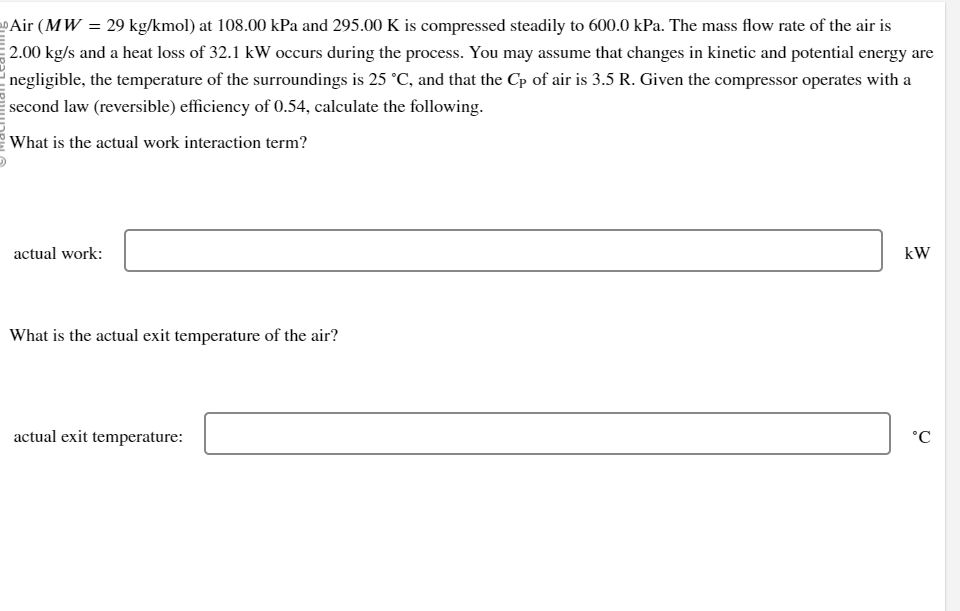
Air ( M W = 2 9 k g k mol ) at 1 0 8 . 0 0 kPa and 2 9 5 .
Air mol at kPa and is compressed steadily to kPa. The mass flow rate of the air is
and a heat loss of occurs during the process. You may assume that changes in kinetic and potential energy are
negligible, the temperature of the surroundings is and that the of air is Given the compressor operates with a
second law reversible efficiency of calculate the following.
What is the actual work interaction term?
actual work:
What is the actual exit temperature of the air?
actual exit temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


