Answered step by step
Verified Expert Solution
Question
1 Approved Answer
All chemical reactions require that certain covalent bonds be broken within the reactants. In order for this to occur, the reactants must have sufficient
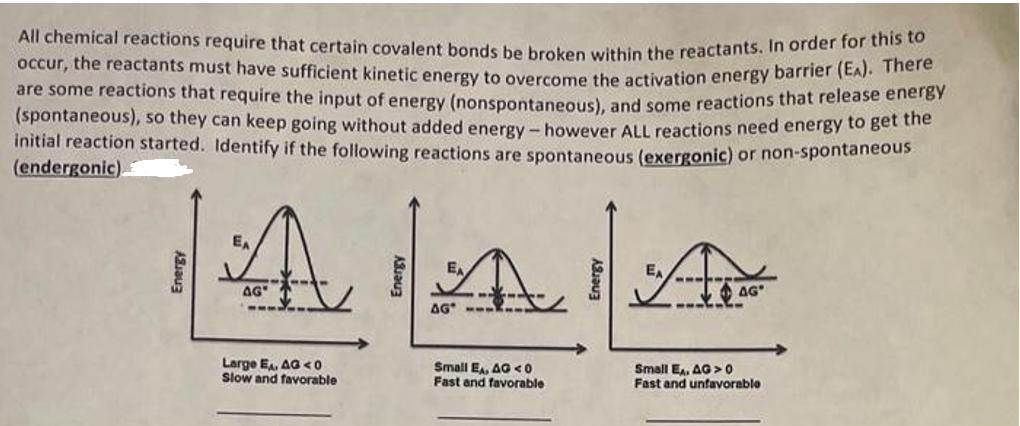
All chemical reactions require that certain covalent bonds be broken within the reactants. In order for this to occur, the reactants must have sufficient kinetic energy to overcome the activation energy barrier (EA). There are some reactions that require the input of energy (nonspontaneous), and some reactions that release energy (spontaneous), so they can keep going without added energy - however ALL reactions need energy to get the initial reaction started. Identify if the following reactions are spontaneous (exergonic) or non-spontaneous (endergonic) Energy A AG" Energy ---, AA AG Large E, AG 0 Fast and unfavorable
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution Step1 1 The first reaction with large activation energy EA and negative G ie G 0 is spontan...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


