Answered step by step
Verified Expert Solution
Question
1 Approved Answer
all of these questions are related constants Periodic Tau Part A Write condensed electron configuration for S. Express your answer in condensed form, in order
all of these questions are related 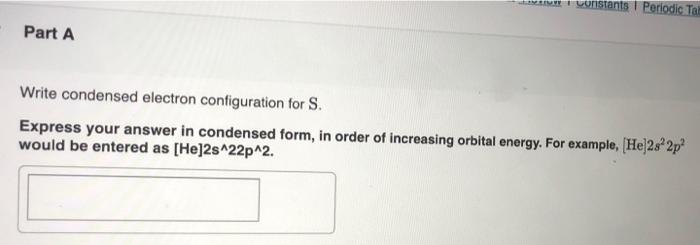
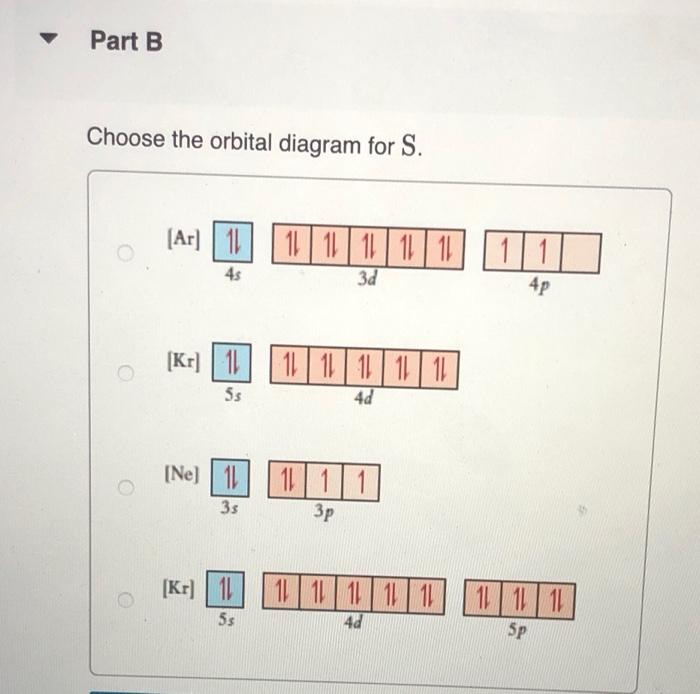
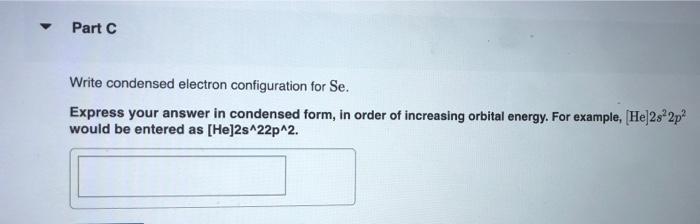
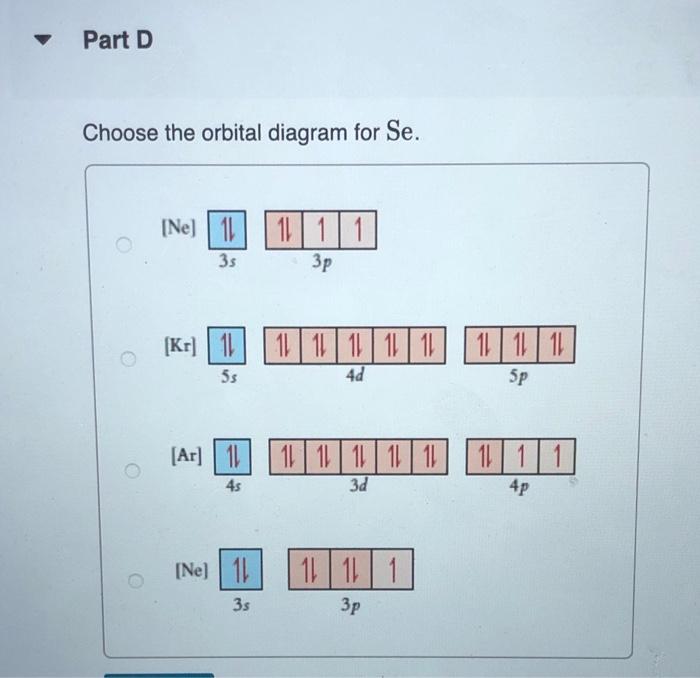
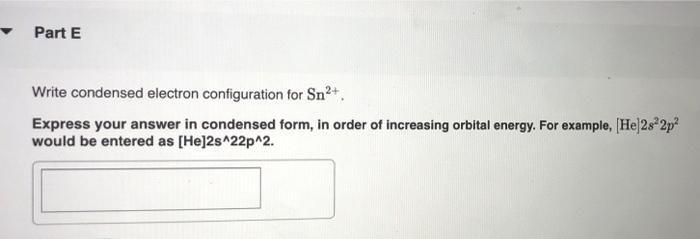
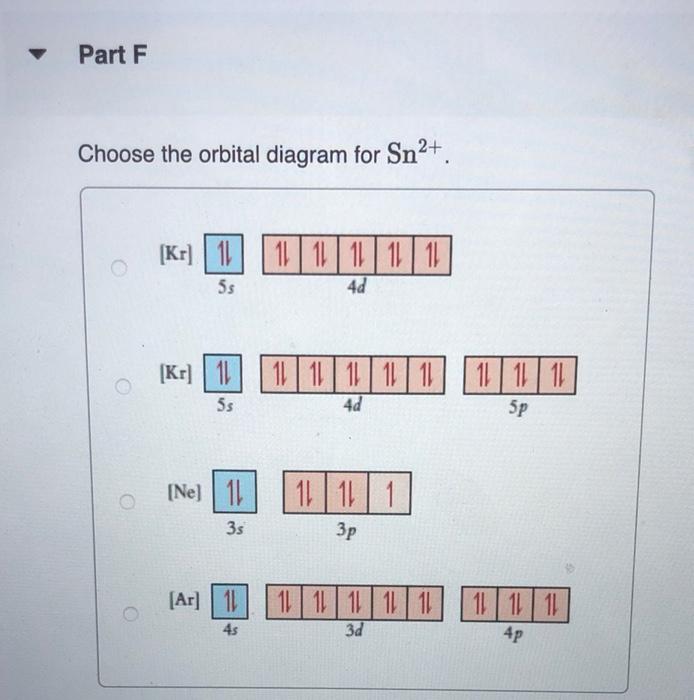
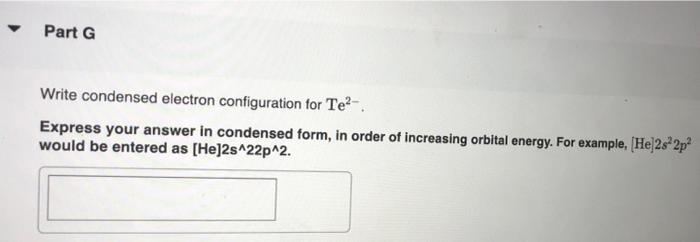
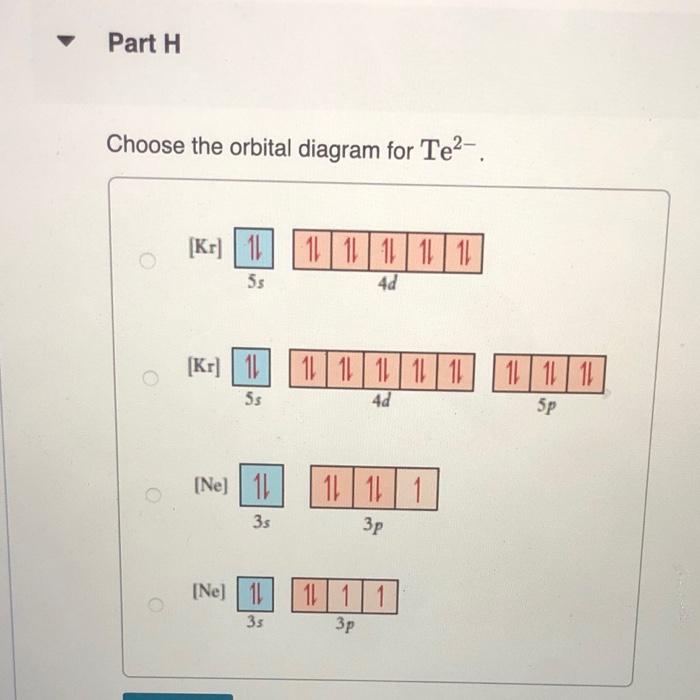
constants Periodic Tau Part A Write condensed electron configuration for S. Express your answer in condensed form, in order of increasing orbital energy. For example, He]2s22p? would be entered as [He]2s22p^2. v Part B Choose the orbital diagram for S. (Ar) 11 11111111111 11 4p 3d [Kr) 1 1111111111 5s 4d [Ne) 11 3s 3p [Kr) 11 111111111111 5s 40 Sp Part C Write condensed electron configuration for Se. Express your answer in condensed form, in order of increasing orbital energy. For example, (He|2s22p would be entered as [He]2s^22p^2. Part D Choose the orbital diagram for Se. [Ne) 111111 3p 35 ] [Kr) 11 11 11 11 111111 5s 4d 5p [Ar] 11 1 1 1 1 1 1 1 1 111111 3d 45 2 4p [Ne) 16 11111 35 Part E Write condensed electron configuration for Sn2+. Express your answer in condensed form, in order of increasing orbital energy. For example, (He)2822p? would be entered as [He]2s22p^2. Part F Choose the orbital diagram for Sn2+. 2+ [Kr] 111111111111 4d 55 [KR] 11111111111111 Kr 5s 4d 5p [Ne) 11 3s [Ar] 11 ] 111111111111 4s 3d 4p Part 6 Write condensed electron configuration for Te- Express your answer in condensed form, in order of increasing orbital energy. For example, (He 23*2p? would be entered as [He]2s^22p^2. Part 1 Choose the orbital diagram for Tez- [Kr] 111111111111 5s 4d [Kr] 1 11111111 1111 5s 4d 5p [Ne) 11 111 3p 3s [Nel 1 1 11 2 35 3p 







Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


