Answered step by step
Verified Expert Solution
Question
1 Approved Answer
all one question. . P 4 E F P 3 Pressure P2 B. P G A T. T3 TA Temperature Variable Ty T2 T3 TA
all one question. 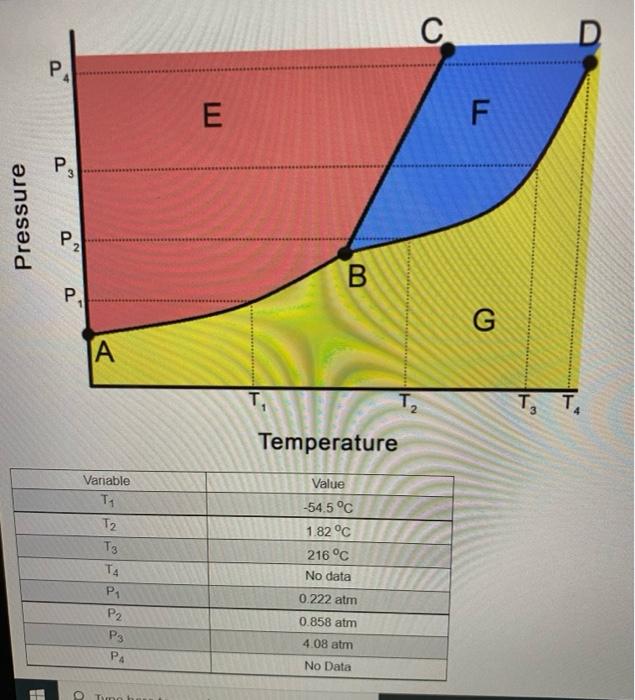
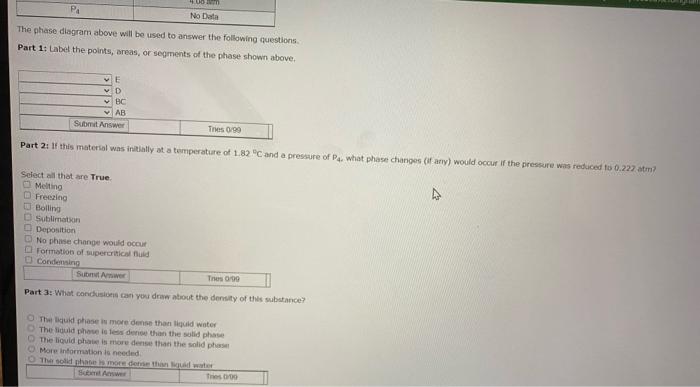
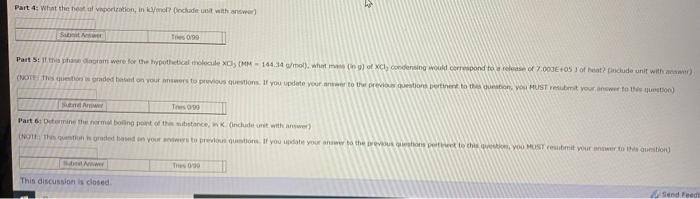
. P 4 E F P 3 Pressure P2 B. P G A T. T3 TA Temperature Variable Ty T2 T3 TA . P2 P3 P4 Value -54.5 C 1.82 C 216 C No data 0.222 atm 0.858 atm 4.08 atm No Data 1 To 100 PA No Data The phase diagram above will be used to answer the following questions Part 1: label the points, areas, or segments of the phase shown above. VE VD BC AB Submit Answer Tres 0/99 Part 2: If this material was initially at a temperature of 1.82 C and a pressure of what phase changes (if any) would occur if the pressure was reduced to 0.227 am Select all that are True Melting Freezing Bolling Sublimation Deposition No phase change would occur O Formation of supercritical uld Condensing Sube Thes Part 3: What condusions can you draw about the density of this substance The quid phase is more dense than oud water The liquid phone is les dense than the solid phase The liquid phase is more dense than the solid phase More information is needed SA Part 4: What the total vaporization, in ko? Decadent with answer) Sub T0190 Part 5: i tha phanagrams were for the hypothetical molecule CCM - 144.34 /mol), what man of XI, condensing would correspond to release of 2.000E05 of eat include unit with (NOTE These graded on your wors to previous questions. If you update your to the previsione per the MUST be your not) Sen II Tres Part 6 termine the bling the stance, include unit with otthon med barn your previous If you be your new to the one to this YOU MUST bmit your het www The 90 This discussion is closed Send Feed 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


