Answered step by step
Verified Expert Solution
Question
1 Approved Answer
all pleasssse Card 6. 1. Calculate AH, ASO, AG and T of equilibrium for the reaction: CH4(g) + 2O2(g) + CO2(g) + 2H2O(l). Substance AH(kJ/mol)
all pleasssse 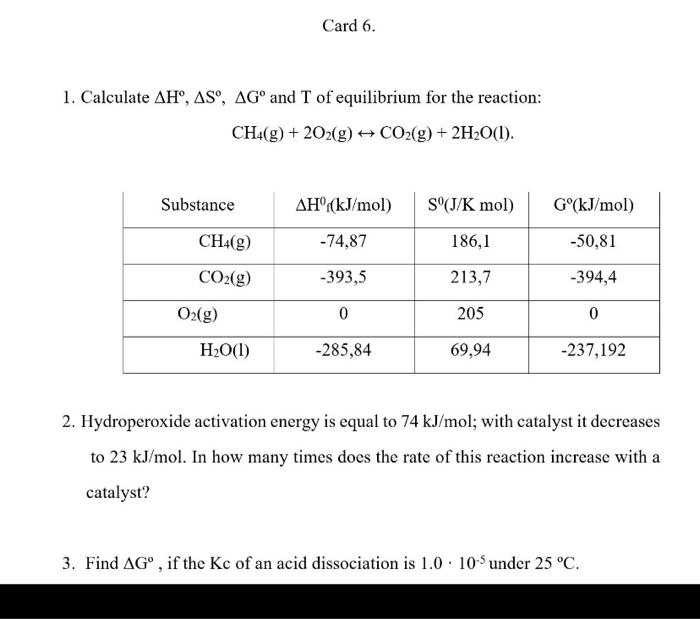
Card 6. 1. Calculate AH, ASO, AG and T of equilibrium for the reaction: CH4(g) + 2O2(g) + CO2(g) + 2H2O(l). Substance AH(kJ/mol) S'(J/K mol) G(kJ/mol) CH4g) -74,87 186,1 -50,81 CO2(g) -393,5 213,7 -394,4 O2(g) 0 205 0 H2O(1) -285,84 69,94 -237,192 2. Hydroperoxide activation energy is equal to 74 kJ/mol; with catalyst it decreases to 23 kJ/mol. In how many times does the rate of this reaction increase with a catalyst? 3. Find AG , if the Kc of an acid dissociation is 1.0 10 under 25 C 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


