Answered step by step
Verified Expert Solution
Question
1 Approved Answer
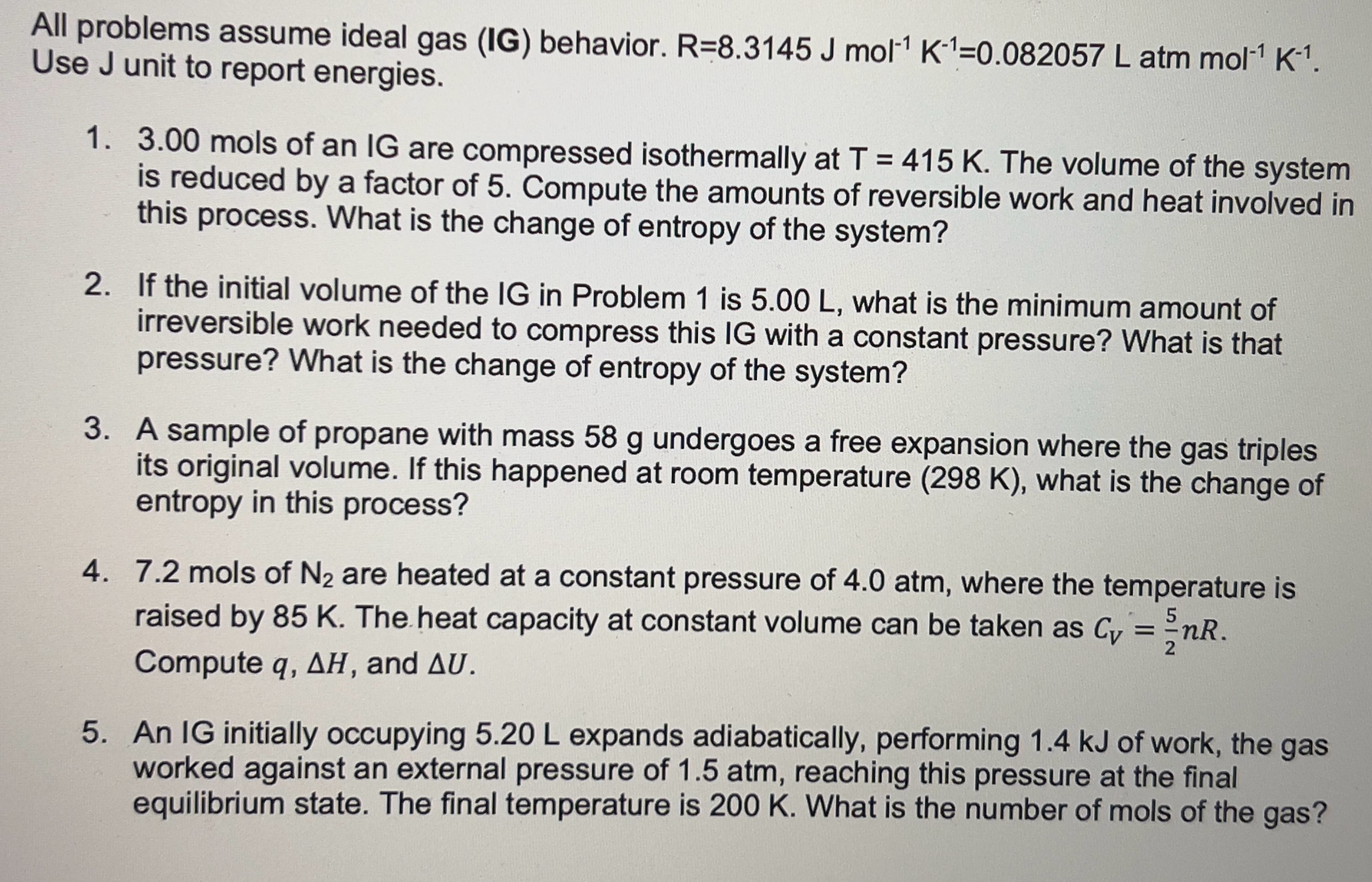
All problems assume ideal gas ( IG ) behavior. R = 8 . 3 1 4 5 J m o l - 1 K -
All problems assume ideal gas IG behavior.
Use J unit to report energies.
mols of an IG are compressed isothermally at The volume of the system
is reduced by a factor of Compute the amounts of reversible work and heat involved in
this process. What is the change of entropy of the system?
If the initial volume of the IG in Problem is what is the minimum amount of
irreversible work needed to compress this IG with a constant pressure? What is that
pressure? What is the change of entropy of the system?
A sample of propane with mass undergoes a free expansion where the gas triples
its original volume. If this happened at room temperature what is the change of
entropy in this process?
mols of are heated at a constant pressure of atm, where the temperature is
raised by The heat capacity at constant volume can be taken as
Compute and
An IG initially occupying expands adiabatically, performing of work, the gas
worked against an external pressure of atm, reaching this pressure at the final
equilibrium state. The final temperature is What is the number of mols of the gas?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


