Answered step by step
Verified Expert Solution
Question
1 Approved Answer
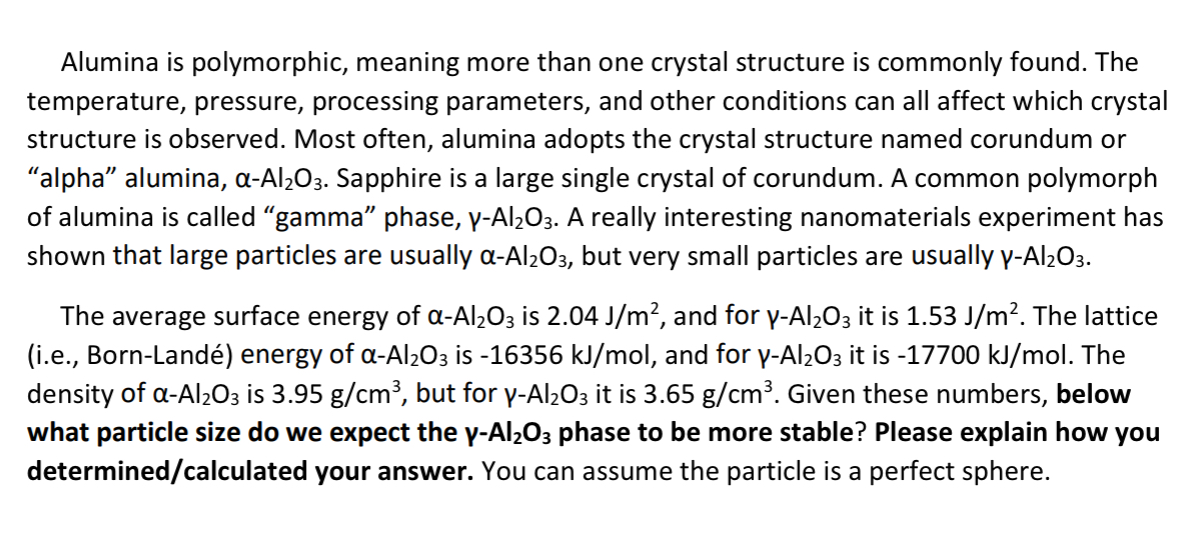
Alumina is polymorphic, meaning more than one crystal structure is commonly found. The temperature, pressure, processing parameters, and other conditions can all affect which crystal
Alumina is polymorphic, meaning more than one crystal structure is commonly found. The
temperature, pressure, processing parameters, and other conditions can all affect which crystal
structure is observed. Most often, alumina adopts the crystal structure named corundum or
"alpha" alumina, Sapphire is a large single crystal of corundum. A common polymorph
of alumina is called "gamma" phase, A really interesting nanomaterials experiment has
shown that large particles are usually but very small particles are usually
The average surface energy of is and for it is The lattice
ie BornLand energy of is and for it is The
density of is but for it is Given these numbers, below
what particle size do we expect the phase to be more stable? Please explain how you
determinedcalculated your answer. You can assume the particle is a perfect sphere.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


