Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure : Cl-N-Cl: : 0: : Cl: :0 ** -0: H= Br ww Is
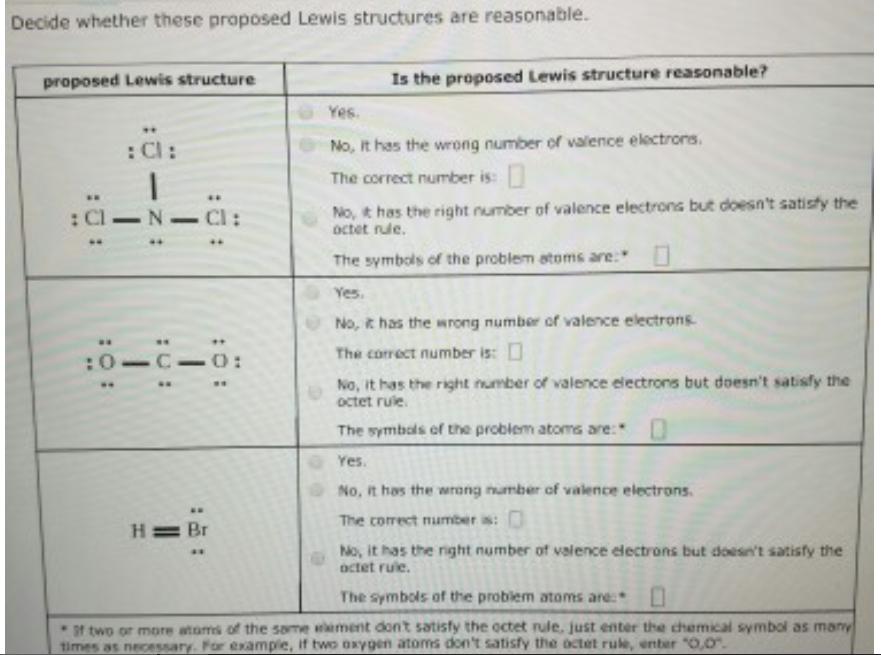
Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure : Cl-N-Cl: : 0: : Cl: :0 ** -0: H= Br ww Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem stoms are: Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: 0 If two or more atoms of the same wement don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".
Step by Step Solution
★★★★★
3.51 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Solution Q1 Answer Yes Expl...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


