Answered step by step
Verified Expert Solution
Question
1 Approved Answer
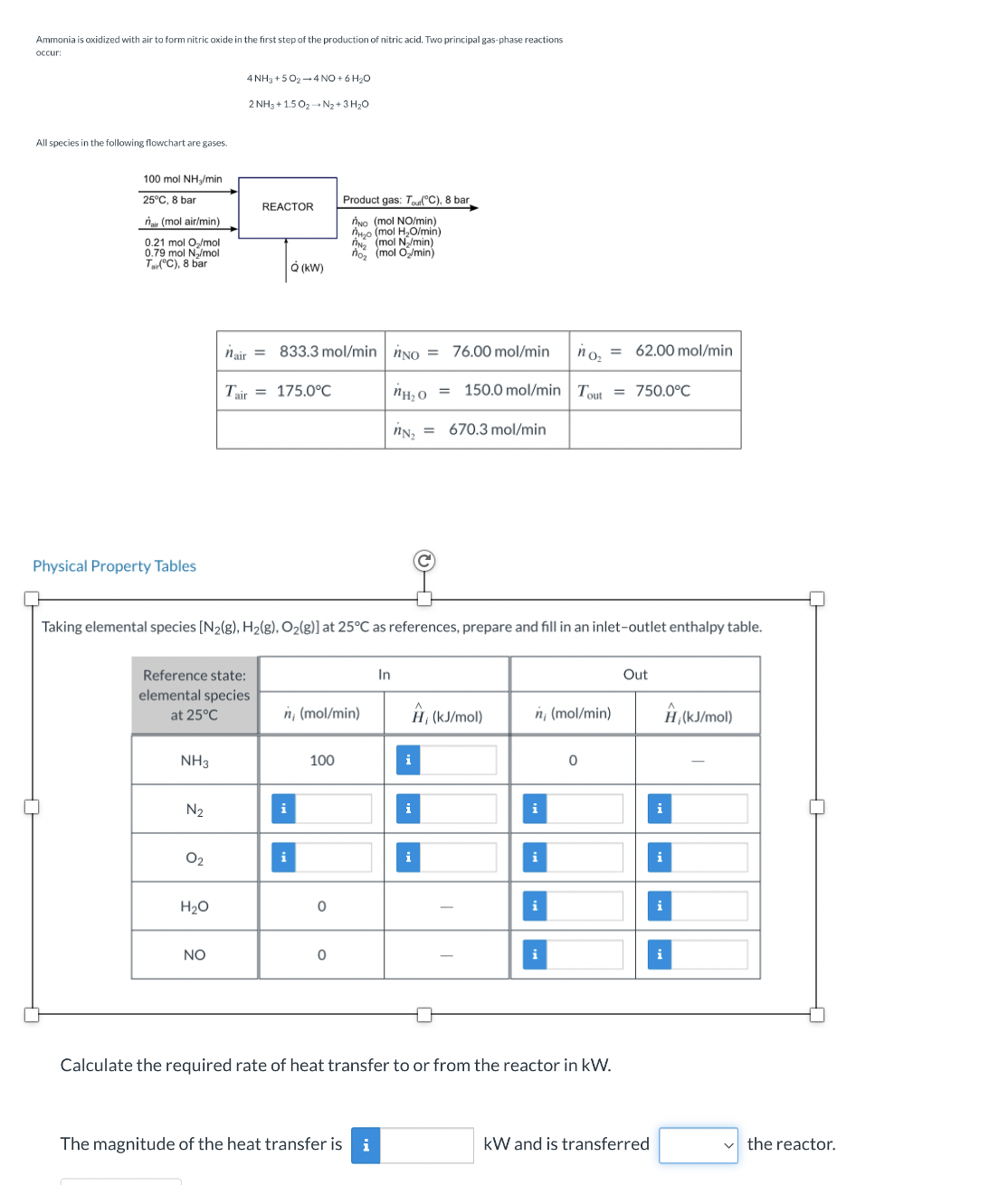
Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Two principal gas - phase reactions
Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Two principal gasphase reactions
occur:
All species in the following flowchart are gases.
Physical Property Tables
Taking elemental species at as references, prepare and fill in an inletoutlet enthalpy table.
Calculate the required rate of heat transfer to or from the reactor in
The magnitude of the heat transfer is
and is transferred
the reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


