Question
Ammonium hydrogen sulfide, NH4HS(s), is an unstable compound that readily decomposes into ammonia, NH3(g), and hydrogen sulfide, H2S(g). The following thermodynamic data are known at
Ammonium hydrogen sulfide, NH4HS(s), is an unstable compound that readily decomposes into ammonia, NH3(g), and hydrogen sulfide, H2S(g). The following thermodynamic data are known at 25 C a) Justify with calculations at 25 oC, if under the indicated conditions the process is exothermic or endothermic. b) Justify with calculations at 25 oC, if under the indicated conditions the system is ordered or disordered. c) Justify with calculations at 25 oC, if under the indicated conditions the system is spontaneous or non-spontaneous.
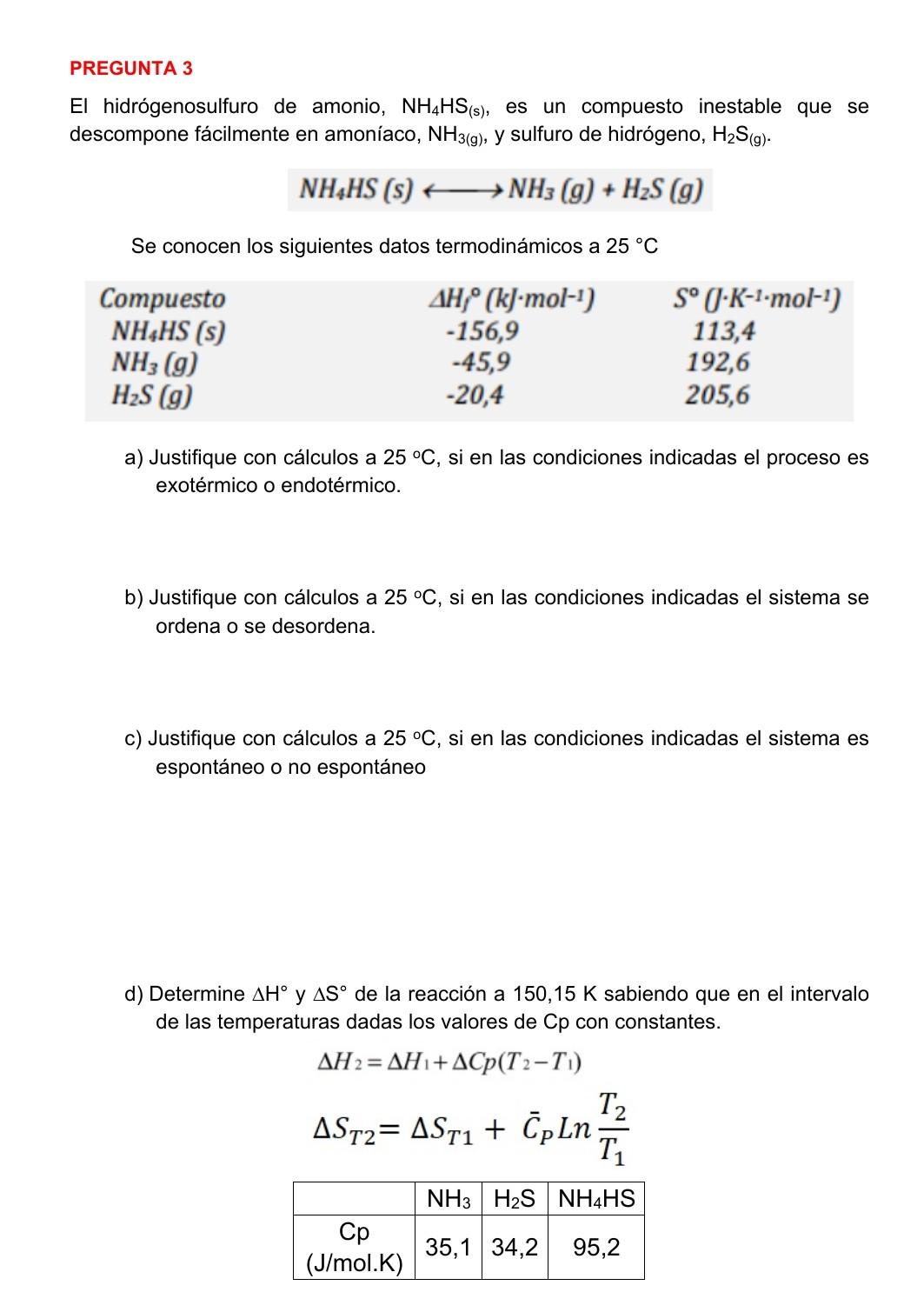
d) Determine H and S of the reaction at 150.15 K knowing that in the interval of the given temperatures the values of Cp with constants. NH3 H2S NH4HS Cp (J/mol.K) 35.1 34.2 95.2
PREGUNTA 3 El hidrgenosulfuro de amonio, NH4HS(s), es un compuesto inestable que se descompone fcilmente en amonaco, NH3(g), y sulfuro de hidrgeno, H2S(g). NH4HS (s) + + NH3(g) + H2S (9) Se conocen los siguientes datos termodinmicos a 25 C Compuesto NH4HS (S) NH3 (9) H2S (9) AH, (kg-mol-1) -156,9 -45,9 -20,4 S (j-K-1-mol-1) 113,4 192,6 205,6 a) Justifique con clculos a 25 C, si en las condiciones indicadas el proceso es exotrmico o endotrmico. b) Justifique con clculos a 25 C, si en las condiciones indicadas el sistema se ordena o se desordena. c) Justifique con clculos a 25 C, si en las condiciones indicadas el sistema es espontneo o no espontneo d) Determine AH AS de la reaccin a 150,15 K sabiendo que en el intervalo de las temperaturas dadas los valores de Cp con constantes. AH 2 = AHI+ACp(T2-T1) T2 AST2= AST + Cpln T NH3 H2S NH4HS (J/mol.K) 35,134,2 95,2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


