Question
Ammonium phosphate ((NH,) PO4 is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid (H, PO,) with
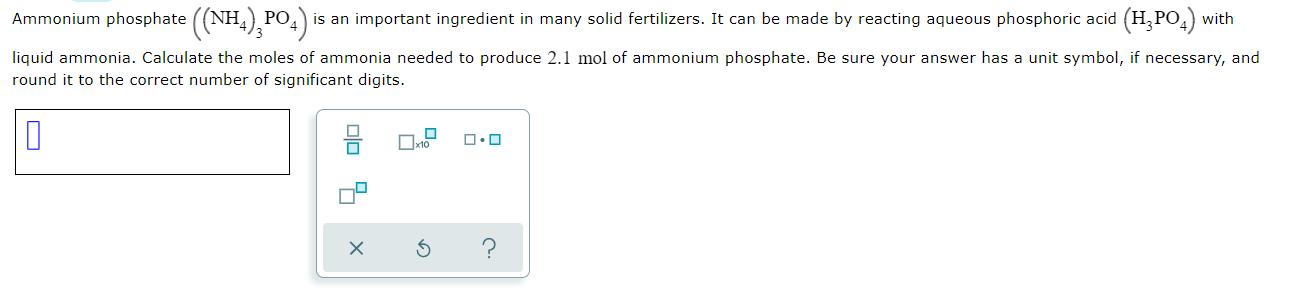
Ammonium phosphate ((NH,) PO4 is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid (H, PO,) with liquid ammonia. Calculate the moles of ammonia needed to produce 2.1 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
fns Balance phophoric the chemical Yeaction betuoen aqueas acid ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



