Question
An analytical chemist is titrating 168.4 mL of a 0.7600M solution of aniline (C,H,NH,) with a 0.8100M solution of HIO,. The p K, of
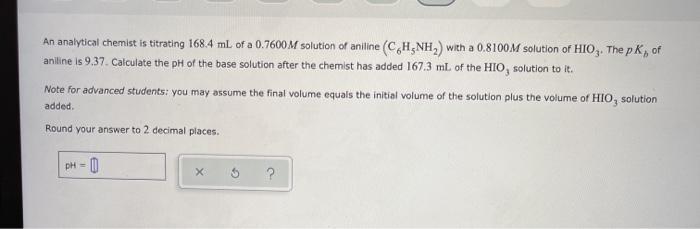
An analytical chemist is titrating 168.4 mL of a 0.7600M solution of aniline (C,H,NH,) with a 0.8100M solution of HIO,. The p K, of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 167.3 mL of the HIO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HIO, solution added. Round your answer to 2 decimal places. pH = 0 %3!
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Final...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



