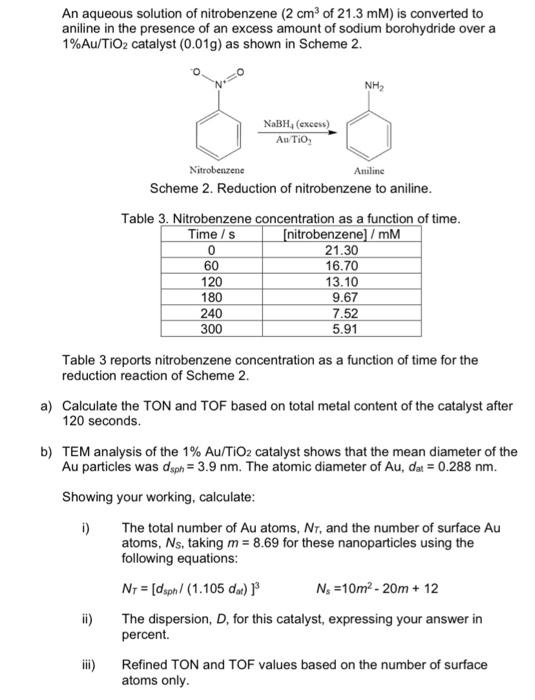
An aqueous solution of nitrobenzene (2 cm of 21.3 mm) is converted to aniline in the presence of an excess amount of sodium borohydride over a 1%Au/TiO2 catalyst (0.019) as shown in Scheme 2. NH NaBH, (excess) Au TiO Nitrobenzene Aniline Scheme 2. Reduction of nitrobenzene to aniline. Table 3. Nitrobenzene concentration as a function of time. Time / s (nitrobenzene] /mM 0 21.30 60 16.70 120 13.10 180 9.67 240 7.52 300 5.91 Table 3 reports nitrobenzene concentration as a function of time for the reduction reaction of Scheme 2. a) Calculate the TON and TOF based on total metal content of the catalyst after 120 seconds. b) TEM analysis of the 1% Au/TiO2 catalyst shows that the mean diameter of the Au particles was dsph= 3.9 nm. The atomic diameter of Au, dat = 0.288 nm. Showing your working, calculate: i) The total number of Au atoms, NT, and the number of surface Au atoms, Ns, taking m = 8.69 for these nanoparticles using the following equations: Nr = [dsph/ (1.105 dar) N. =10m2-20m + 12 ii) The dispersion, D, for this catalyst, expressing your answer in percent. Refined TON and TOF values based on the number of surface atoms only An aqueous solution of nitrobenzene (2 cm of 21.3 mm) is converted to aniline in the presence of an excess amount of sodium borohydride over a 1%Au/TiO2 catalyst (0.019) as shown in Scheme 2. NH NaBH, (excess) Au TiO Nitrobenzene Aniline Scheme 2. Reduction of nitrobenzene to aniline. Table 3. Nitrobenzene concentration as a function of time. Time / s (nitrobenzene] /mM 0 21.30 60 16.70 120 13.10 180 9.67 240 7.52 300 5.91 Table 3 reports nitrobenzene concentration as a function of time for the reduction reaction of Scheme 2. a) Calculate the TON and TOF based on total metal content of the catalyst after 120 seconds. b) TEM analysis of the 1% Au/TiO2 catalyst shows that the mean diameter of the Au particles was dsph= 3.9 nm. The atomic diameter of Au, dat = 0.288 nm. Showing your working, calculate: i) The total number of Au atoms, NT, and the number of surface Au atoms, Ns, taking m = 8.69 for these nanoparticles using the following equations: Nr = [dsph/ (1.105 dar) N. =10m2-20m + 12 ii) The dispersion, D, for this catalyst, expressing your answer in percent. Refined TON and TOF values based on the number of surface atoms only