Question
An atomic anion with a charge of -1 has the following electron configuration: [He] 2s2p6 What is the chemical symbol for the ion? How
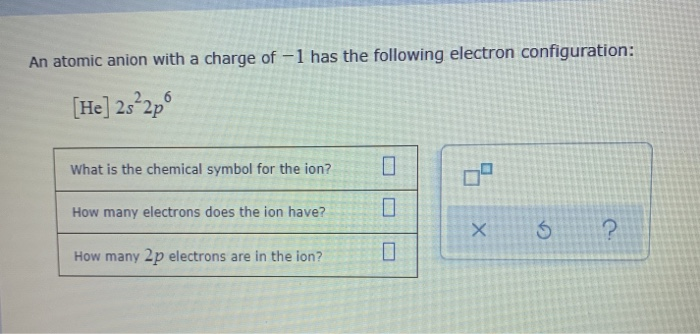
An atomic anion with a charge of -1 has the following electron configuration: [He] 2s2p6 What is the chemical symbol for the ion? How many electrons does the ion have? How many 2p electrons are in the ion? U 0 n X 5 ?
Step by Step Solution
3.28 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1
Answer Answer An atom acquires a negative charge after the addition of an electr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics Principles with Applications
Authors: Douglas C. Giancoli
7th edition
978-0321869111, 321625927, 9780321733627, 321869117, 9780321625922, 321733622, 978-0321762429
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



