Answered step by step
Verified Expert Solution
Question
1 Approved Answer
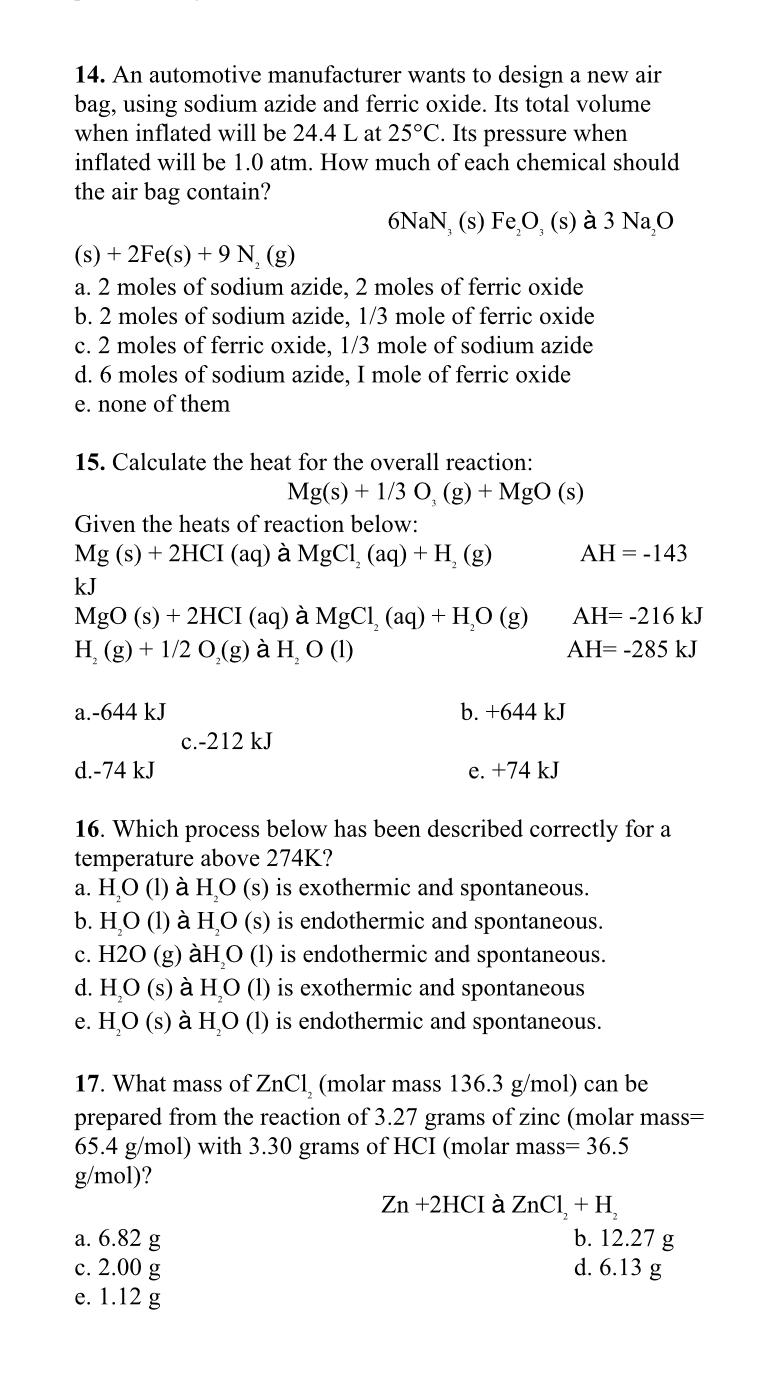
An automotive manufacturer wants to design a new air bag, using sodium azide and ferric oxide. Its total volume when inflated will be 2 4
An automotive manufacturer wants to design a new air bag, using sodium azide and ferric oxide. Its total volume when inflated will be at Its pressure when inflated will be atm. How much of each chemical should the air bag contain?
s
a moles of sodium azide, moles of ferric oxide
b moles of sodium azide, mole of ferric oxide
c moles of ferric oxide, mole of sodium azide
d moles of sodium azide, I mole of ferric oxide
e none of them
Calculate the heat for the overall reaction:
MgO
Given the heats of reaction below:
HCI
MgOHCI
a
b
d
e
Which process below has been described correctly for a temperature above
a is exothermic and spontaneous.
b is endothermic and spontaneous.
c is endothermic and spontaneous.
dl is exothermic and spontaneous
e is endothermic and spontaneous.
What mass of molar mass can be prepared from the reaction of grams of zinc molar mass with grams of HCI molar mass
HCI
a
b
c
d
e
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


