Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An average compact car carries 10 US gallon of gasoline in its fuel tank. The density of gasoline at 20 C is 0.77 g/mL,
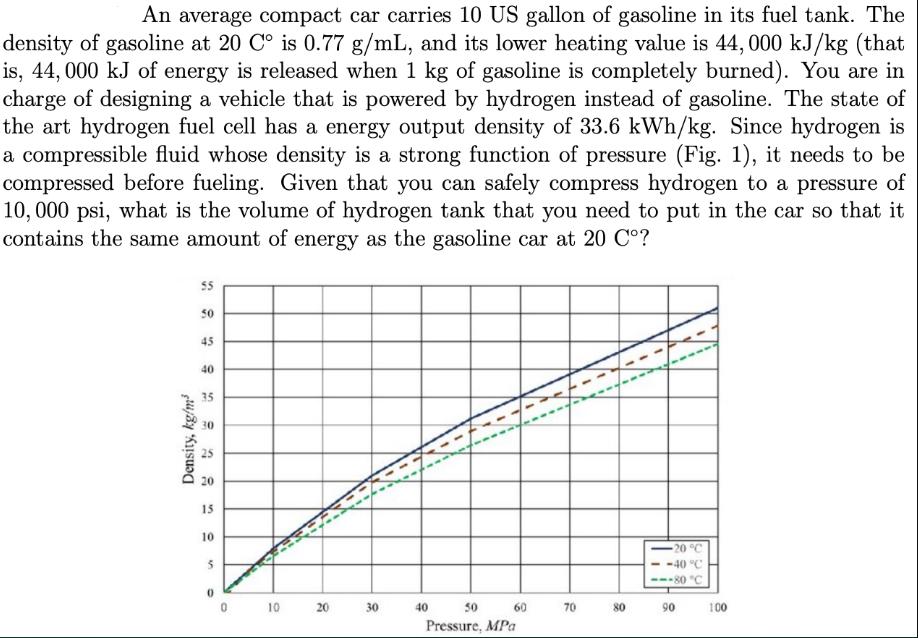
An average compact car carries 10 US gallon of gasoline in its fuel tank. The density of gasoline at 20 C is 0.77 g/mL, and its lower heating value is 44, 000 kJ/kg (that is, 44, 000 kJ of energy is released when 1 kg of gasoline is completely burned). You are in charge of designing a vehicle that is powered by hydrogen instead of gasoline. The state of the art hydrogen fuel cell has a energy output density of 33.6 kWh/kg. Since hydrogen is a compressible fluid whose density is a strong function of pressure (Fig. 1), it needs to be compressed before fueling. Given that you can safely compress hydrogen to a pressure of 10, 000 psi, what is the volume of hydrogen tank that you need to put in the car so that it contains the same amount of energy as the gasoline car at 20 C? Density, kg/m 55 50 45 40 35 20 15 10 5 0 0 10 A 20 30 40 50 Pressure, MPa 60 70 80 -20 C
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer Given The pressure is 10000Pa The energy output density is 336k...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


