Question
An electric current is conducted through water, decomposing it into H and O2 gas, as shown in the reaction below. The reaction takes place
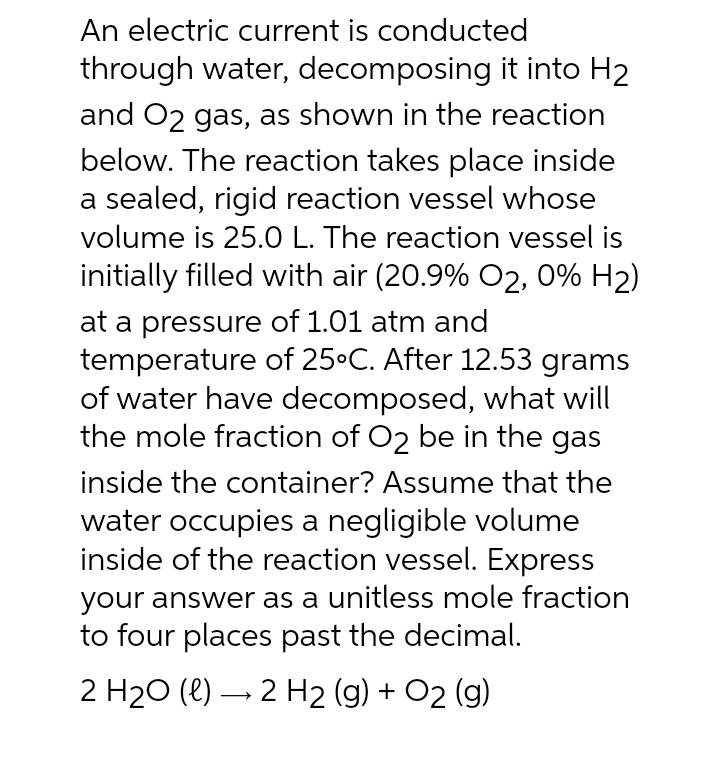
An electric current is conducted through water, decomposing it into H and O2 gas, as shown in the reaction below. The reaction takes place inside a sealed, rigid reaction vessel whose volume is 25.0 L. The reaction vessel is initially filled with air (20.9% O2, 0% H) at a pressure of 1.01 atm and temperature of 25C. After 12.53 grams of water have decomposed, what will the mole fraction of O2 be in the gas inside the container? Assume that the water occupies a negligible volume inside of the reaction vessel. Express your answer as a unitless mole fraction to four places past the decimal. 2 HO (l) 2 H2 (g) + O2 (g)
Step by Step Solution
3.58 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



