Question
An electron in the n = 8 level of a C* ion emits a photon of wavelength 104 nm. Hz (a) What is the
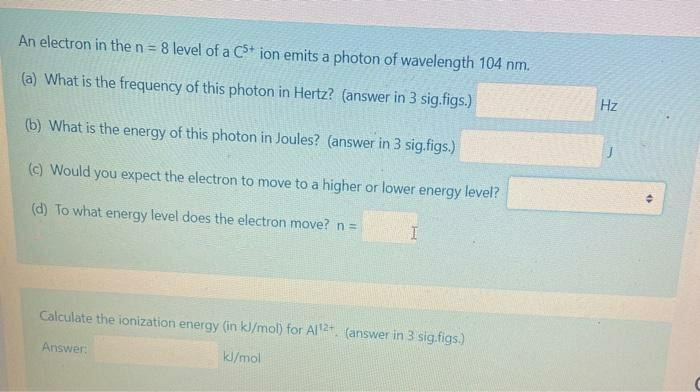
An electron in the n = 8 level of a C* ion emits a photon of wavelength 104 nm. Hz (a) What is the frequency of this photon in Hertz? (answer in 3 sig.figs.) (b) What is the energy of this photon in Joules? (answer in 3 sig.figs.) (c) Would you expect the electron to move to a higher or lower energy level? (d) To what energy level does the electron move? n= Calculate the ionization energy (in kl/mol) for Al2. (answer in 3 sig.figs.) Answer kl/mol
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: Alan Giambattista, Betty Richardson, Robert Richardson
2nd edition
77339681, 978-0077339685
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



