Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An ideal Brayton cycle operates at steady state. The turbine (3-4) and compressor (1-2) are internally reversible and adiabatic, and the heat transfer occurs

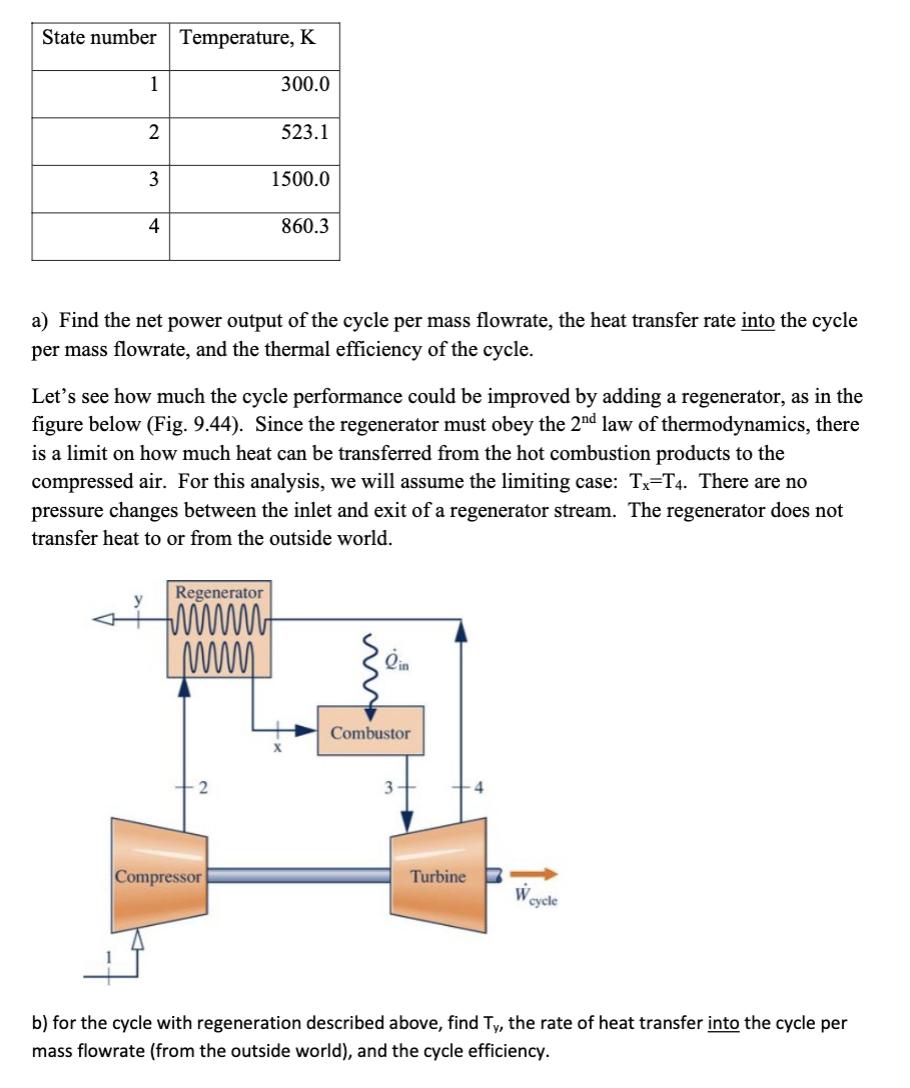
An ideal Brayton cycle operates at steady state. The turbine (3-4) and compressor (1-2) are internally reversible and adiabatic, and the heat transfer occurs at constant pressure. Treat the working fluid contents as an ideal gas with constant specific heats, k=1.4, cp=1004 J/(kg K). All information in this paragraph and in the table below applies to the whole problem. The temperature is listed below for each state: State number Temperature, K 300.0 1 2 3 4 523.1 1500.0 a) Find the net power output of the cycle per mass flowrate, the heat transfer rate into the cycle per mass flowrate, and the thermal efficiency of the cycle. Regenerator timmt www Compressor 860.3 Let's see how much the cycle performance could be improved by adding a regenerator, as in the figure below (Fig. 9.44). Since the regenerator must obey the 2nd law of thermodynamics, there is a limit on how much heat can be transferred from the hot combustion products to the compressed air. For this analysis, we will assume the limiting case: Tx=T4. There are no pressure changes between the inlet and exit of a regenerator stream. The regenerator does not transfer heat to or from the outside world. Combustor 3 Turbine 4 W cycle b) for the cycle with regeneration described above, find Ty, the rate of heat transfer into the cycle per mass flowrate (from the outside world), and the cycle efficiency.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
ANSWER Ste...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


