Question
An ideal dual cycle operates with a mass of 0.25 kg. The dual cycle consists of the following internally reversible processes: 12: adiabatic compression

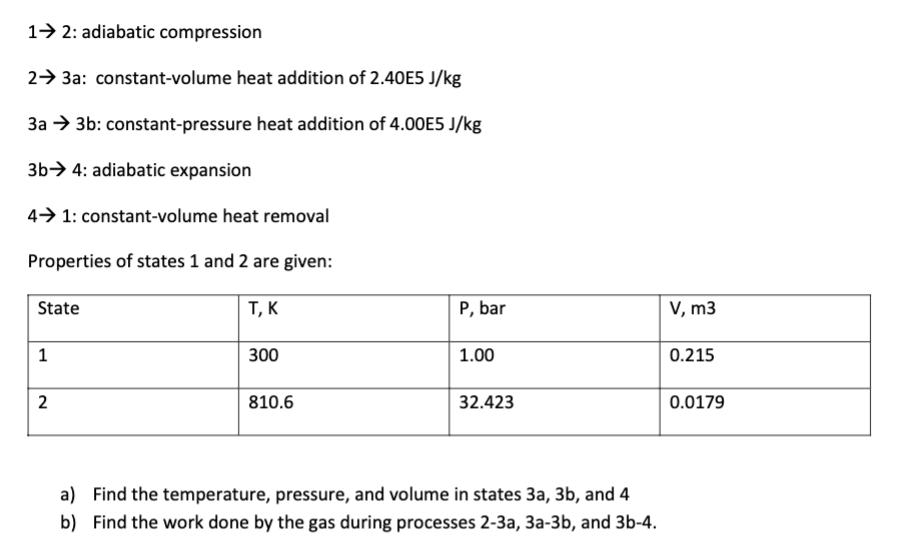
An ideal dual cycle operates with a mass of 0.25 kg. The dual cycle consists of the following internally reversible processes: 12: adiabatic compression 2 3a: constant-volume heat addition of 2.40E5 J/kg 3a 3b: constant-pressure heat addition of 4.00E5 J/kg 3b 4: adiabatic expansion 4 1: constant-volume heat removal Properties of states 1 and 2 are given: State 1 2 T, K 300 810.6 P, bar 1.00 32.423 a) Find the temperature, pressure, and volume in states 3a, 3b, and 4 b) Find the work done by the gas during processes 2-3a, 3a-3b, and 3b-4. V, m3 0.215 0.0179
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles Of Thermodynamics
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
1st Edition
1108426093, 978-1108426091
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



