An ideal gas with a constant pressure heat capacity is compressed from a low pressure (P,) to a higher pressure (P2). The compressor(s) can
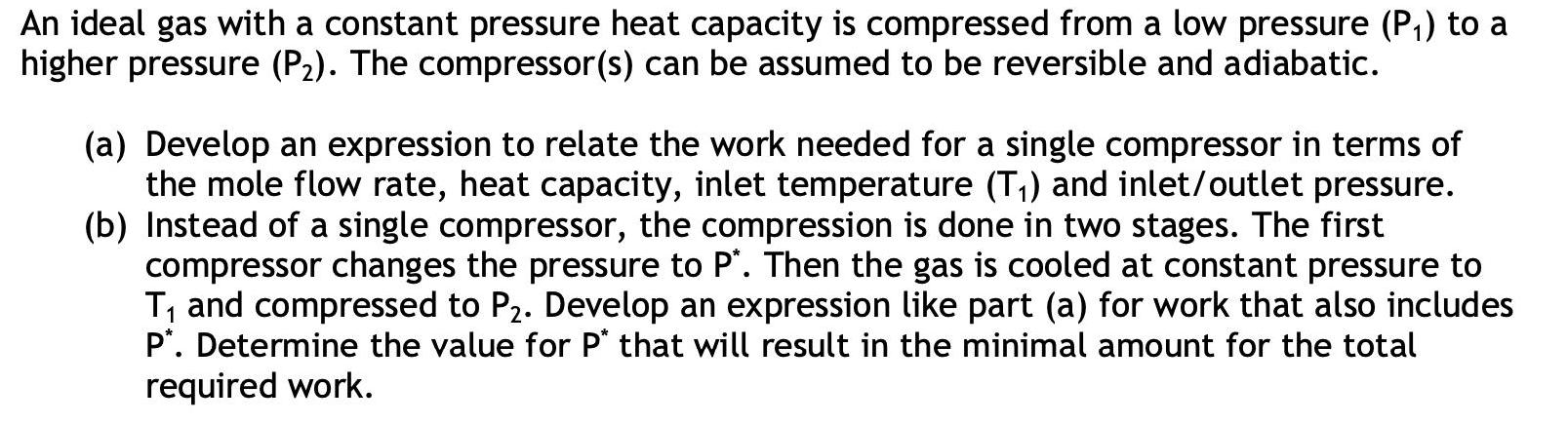
An ideal gas with a constant pressure heat capacity is compressed from a low pressure (P,) to a higher pressure (P2). The compressor(s) can be assumed to be reversible and adiabatic. (a) Develop an expression to relate the work needed for a single compressor in terms of the mole flow rate, heat capacity, inlet temperature (T,) and inlet/outlet pressure. (b) Instead of a single compressor, the compression is done in two stages. The first compressor changes the pressure to P'. Then the gas is cooled at constant pressure to T, and compressed to P2. Develop an expression like part (a) for work that also includes P*. Determine the value for P* that will result in the minimal amount for the total required work.
Step by Step Solution
3.48 Rating (141 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


