Question
An ideal gas with the following composition where T = 260F, P = 1800 psi, V = 1.0 acre ft: Component C1 C2 C3
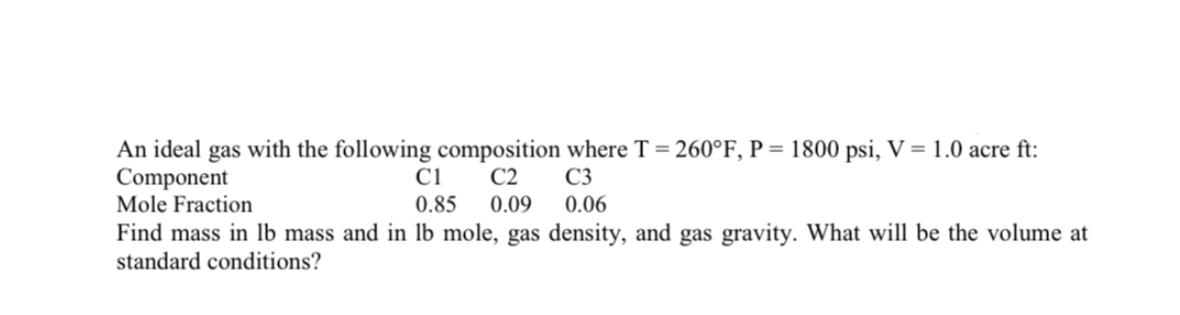
An ideal gas with the following composition where T = 260F, P = 1800 psi, V = 1.0 acre ft: Component C1 C2 C3 0.06 Mole Fraction 0.85 0.09 Find mass in lb mass and in lb mole, gas density, and gas gravity. What will be the volume at standard conditions?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the mass in pounds and moles for each component you can use the ideal gas law PV nRT Where P is pressure V is volume n is the number of moles R is the ideal gas constant and T is temperat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



