Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An insulated beaker with negligible mass contains liquid water with a mass of 0.245 kg and a temperature of 75.9 C. Part A How
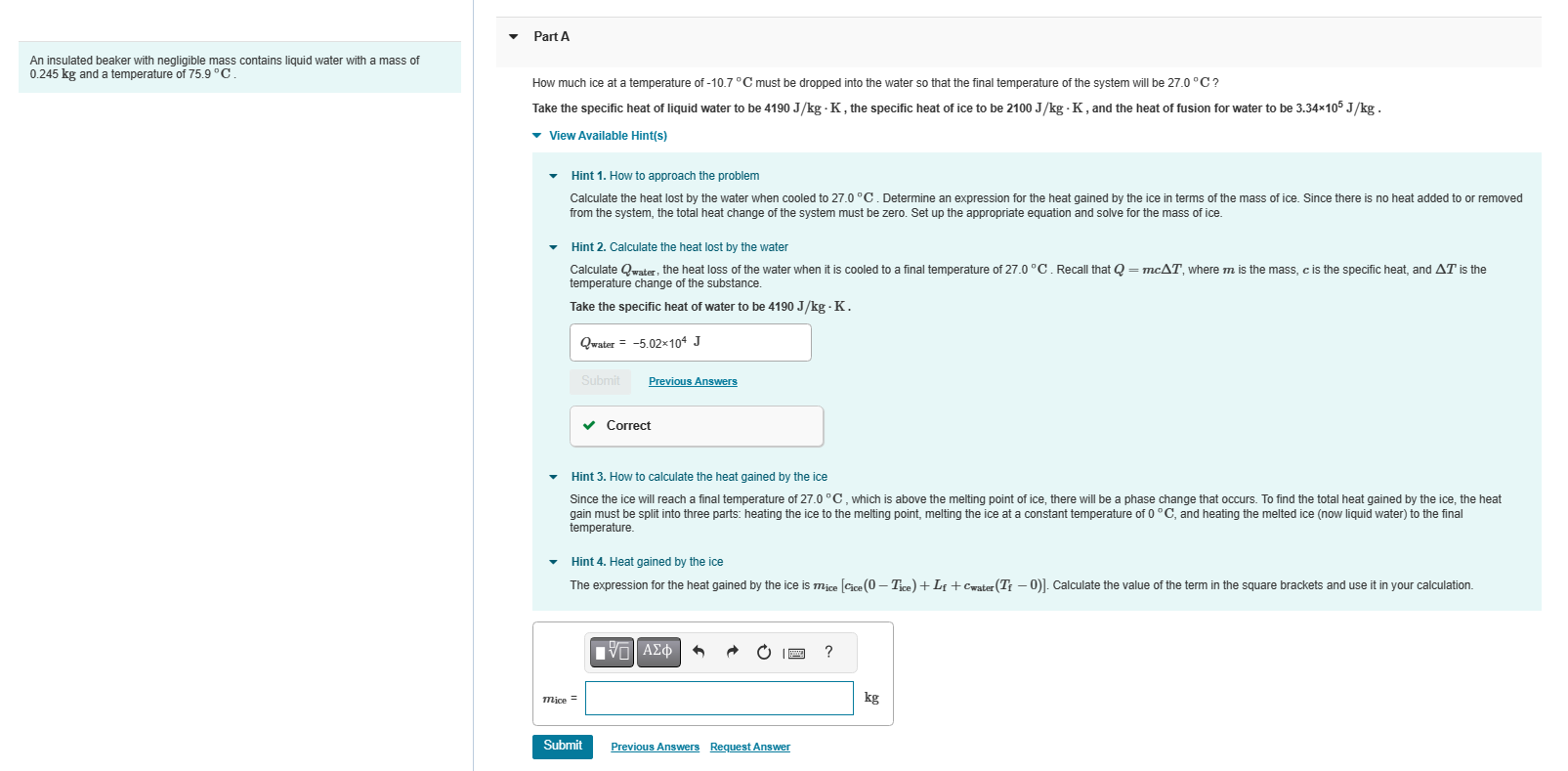
An insulated beaker with negligible mass contains liquid water with a mass of 0.245 kg and a temperature of 75.9 C. Part A How much ice at a temperature of -10.7C must be dropped into the water so that the final temperature of the system will be 27.0 C? Take the specific heat of liquid water to be 4190 J/kg - K, the specific heat of ice to be 2100 J/kg - K, and the heat of fusion for water to be 3.34105 J/kg. View Available Hint(s) Hint 1. How to approach the problem Calculate the heat lost by the water when cooled to 27.0 C. Determine an expression for the heat gained by the ice in terms of the mass of ice. Since there is no heat added to or removed from the system, the total heat change of the system must be zero. Set up the appropriate equation and solve for the mass of ice. Hint 2. Calculate the heat lost by the water Calculate Qwater, the heat loss of the water when it is cooled to a final temperature of 27.0 C. Recall that Q = mcAT, where m is the mass, c is the specific heat, and AT is the temperature change of the substance. Take the specific heat of water to be 4190 J/kg. K. Qwater = -5.02104 J Submit Previous Answers Correct Hint 3. How to calculate the heat gained by the ice Since the ice will reach a final temperature of 27.0C, which is above the melting point of ice, there will be a phase change that occurs. To find the total heat gained by the ice, the heat gain must be split into three parts: heating the ice to the melting point, melting the ice at a constant temperature of 0 C, and heating the melted ice (now liquid water) to the final temperature. mice = Hint 4. Heat gained by the ice The expression for the heat gained by the ice is mice [Cice (0-Tice) + Lf+Cwater (Tf - 0)]. Calculate the value of the term in the square brackets and use it in your calculation. Submit IVE| Ima ? Previous Answers Request Answer kg
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this problem we first calculate the heat lost by the water when cooled to 270C Qlo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


