Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An internal combustion engine uses octane as fuel. The air and fuel vapor mixture enter the engine at 298K. Twenty per cent excess air is
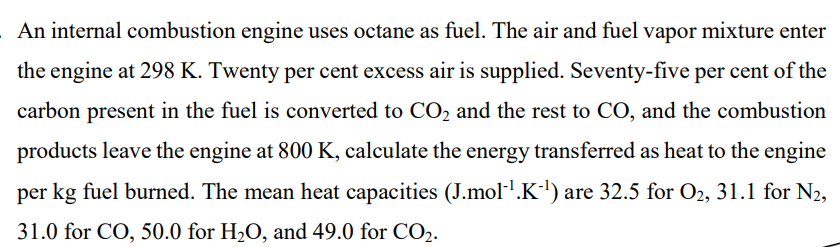 An internal combustion engine uses octane as fuel. The air and fuel vapor mixture enter the engine at 298K. Twenty per cent excess air is supplied. Seventy-five per cent of the carbon present in the fuel is converted to CO2 and the rest to CO, and the combustion products leave the engine at 800K, calculate the energy transferred as heat to the engine per kg fuel burned. The mean heat capacities (J.mol1K1) are 32.5 for O2,31.1 for N2, 31.0 for CO,50.0 for H2O, and 49.0 for CO2
An internal combustion engine uses octane as fuel. The air and fuel vapor mixture enter the engine at 298K. Twenty per cent excess air is supplied. Seventy-five per cent of the carbon present in the fuel is converted to CO2 and the rest to CO, and the combustion products leave the engine at 800K, calculate the energy transferred as heat to the engine per kg fuel burned. The mean heat capacities (J.mol1K1) are 32.5 for O2,31.1 for N2, 31.0 for CO,50.0 for H2O, and 49.0 for CO2 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


