Question
An inventor has noticed that people who have steam heat in their houses often generate a lot of hot water condensate, which is dumped down
An inventor has noticed that people who have steam heat in their houses often generate a lot of hot water condensate, which is dumped down a drain in the basement. He decided to measure the energy content of this stream and found that approximately 50 liters of condensate per hour at a temperature of 95C is generated at the drainpipe. This seems to be lot of energy going down the drain, so he decides to do something to prevent this waste. He decides to use a Carnot engine to turn this energy into work. In this tricky device, (the Carnotcon heat engine) he contacts the hot condensate with cold tap water at 6C, and the streams exit the engine and go down the drain at virtually the same temperature (TD). If the cold tap water has flow of 60 liters per hour, please perform the following calculations for the inventor to see if he is on the right track. Assume constant heat capacities for all streams, where: CP water = 4.18 KJ/Kg*K.
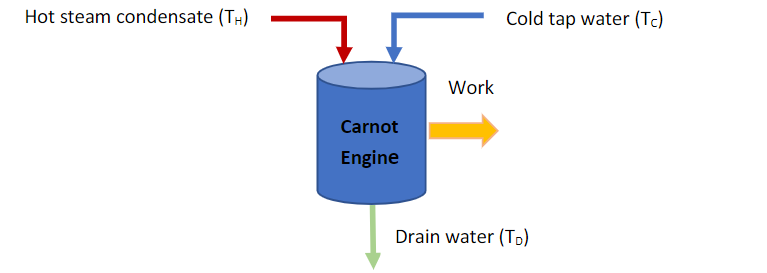
(a) What is the temperature of the drain water (TD)? (b) How much power would a Carnot engine produce under these circumstances? (c) Could you suggest a tap water flowrate that would improve the work produced? (d) What is the Carnot efficiency of this device? (e) What is the entropy & enthalpy change of the hot water? How about the cold water? (f) What is the overall entropy change of the streams of this device? (g) How would the work produced change if we didnt completely condense the steam but instead used saturated steam at 105C and 40 kg/hr on the hot side with adequate cooling water to produce drain water at 40C?
Hot steam condensate (TH) Cold tap water (TC) Work Carnot Engine Drain water (TO) Hot steam condensate (TH) Cold tap water (TC) Work Carnot Engine Drain water (TO)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


