Answered step by step
Verified Expert Solution
Question
1 Approved Answer
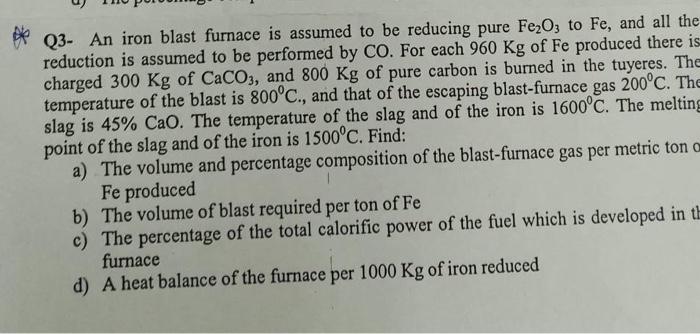
An iron blast furnace is assumed to be reducing pure FezO, to Fe, and all the reduction is assumed to be performed by CO. For
An iron blast furnace is assumed to be reducing pure FezO, to Fe, and all the
reduction is assumed to be performed by CO. For each 960 Kg of Fe produced there is charged 300 Kg of CaCO3, and 800 Kg of pure carbon is burned in the tuyeres. The temperature of the blast is 800C., and that of the escaping blast-furnace gas 200C. The slag is 45% CaO. The temperature of the slag and of the iron is 1600C. The melting point of the slag and of the iron is 1500C. Find:
- The volume and percentage composition of the blast-furnace gas per metric ton of Fe produced
- The volume of blast required per ton of Fe
- The percentage of the total calorific power of the fuel which is developed in the furnace
- A heat balance of the furnace per 1000 Kg of iron reduced
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


