Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An isothermal gas-phase decomposition reaction is given by Eq. 1: A B C (Eq. 1) The reaction is set at 300 F and 10
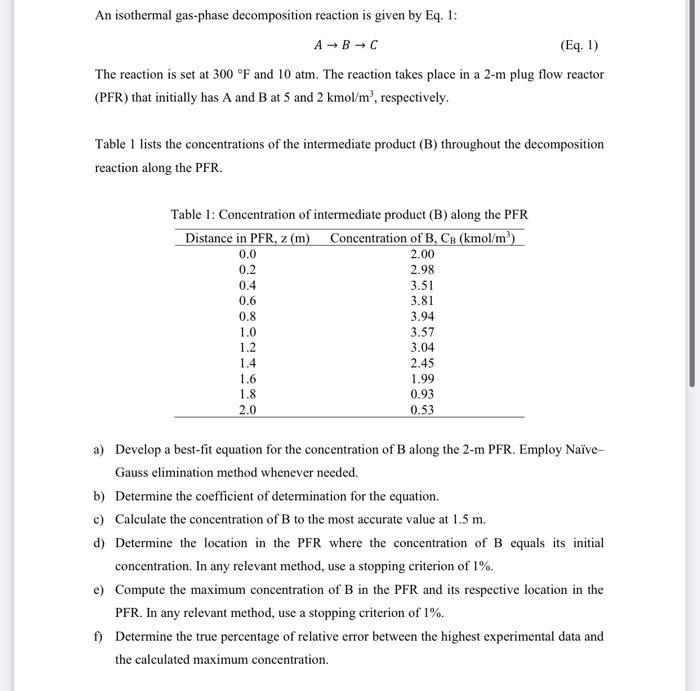
An isothermal gas-phase decomposition reaction is given by Eq. 1: A B C (Eq. 1) The reaction is set at 300 F and 10 atm. The reaction takes place in a 2-m plug flow reactor (PFR) that initially has A and B at 5 and 2 kmol/m, respectively. Table 1 lists the concentrations of the intermediate product (B) throughout the decomposition reaction along the PFR. Table 1: Concentration of intermediate product (B) along the PFR Distance in PFR, z (m) Concentration of B, CB (kmol/m) 0.0 2.00 2.98 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 3.51 3.81 3.94 3.57 3.04 2.45 1.99 0.93 0.53 a) Develop a best-fit equation for the concentration of B along the 2-m PFR. Employ Nave- Gauss elimination method whenever needed. b) Determine the coefficient of determination for the equation. c) Calculate the concentration of B to the most accurate value at 1.5 m. d) Determine the location in the PFR where the concentration of B equals its initial concentration. In any relevant method, use a stopping criterion of 1%. e) Compute the maximum concentration of B in the PFR and its respective location in the PFR. In any relevant method, use a stopping criterion of 1%. f) Determine the true percentage of relative error between the highest experimental data and the calculated maximum concentration.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The complete a...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


