Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If a sample contains 1.1 g of Bi-210 (atomic mass=209.984105 amu), how many beta emissions would occur in 13.3 days? Express your answer using
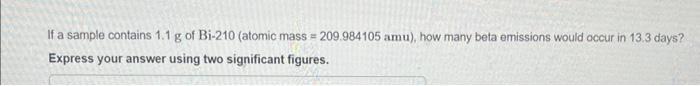

If a sample contains 1.1 g of Bi-210 (atomic mass=209.984105 amu), how many beta emissions would occur in 13.3 days? Express your answer using two significant figures. P Bismuth-210 is beta emitter with a half-life of 5.0 days.
Step by Step Solution
★★★★★
3.37 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
The halflife of a first order reaction can be found out using the relation t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635e32ab79be2_182279.pdf
180 KBs PDF File
635e32ab79be2_182279.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


