Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An organic liquid, linear alkyl benzene, LAB and sulfuric acid (H,So,) are continuously fed to a laboratory reactor (baffled mixing tank). Volumetric flow rate
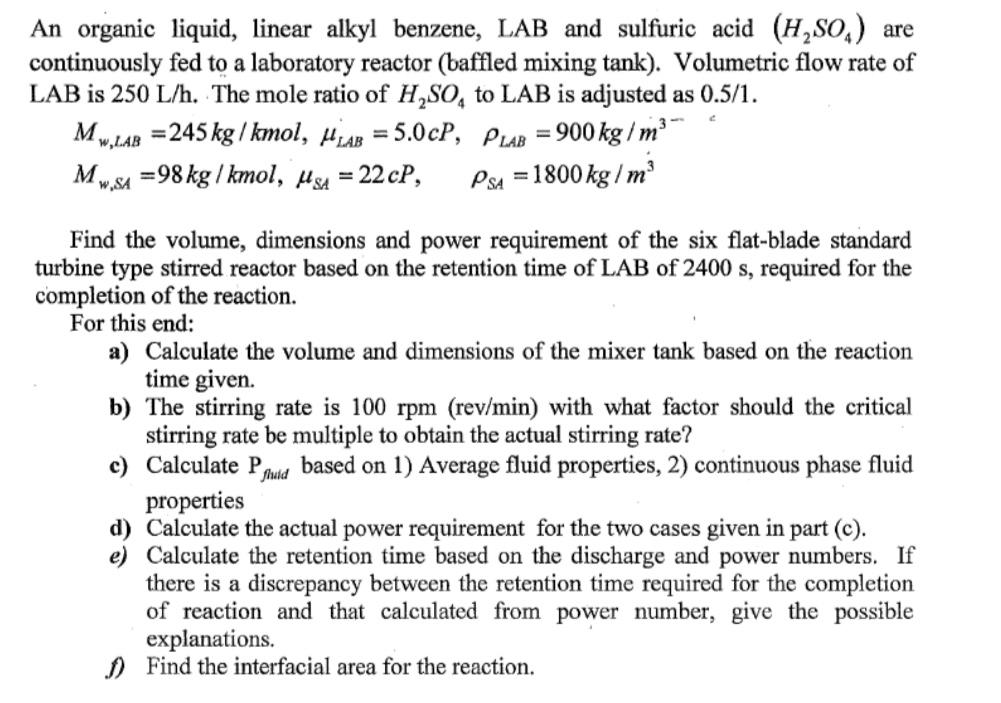
An organic liquid, linear alkyl benzene, LAB and sulfuric acid (H,So,) are continuously fed to a laboratory reactor (baffled mixing tank). Volumetric flow rate of LAB is 250 L/h. The mole ratio of H,SO, to LAB is adjusted as 0.5/1. M. w,LAB =245 kg / kmol, HLAB = 5.0cP, PLAB = 900 kg/m %3D %3D =98 kg / kmol, HgA = 22 cP, PsA = 1800 kg / m w,SA Find the volume, dimensions and power requirement of the six flat-blade standard turbine type stirred reactor based on the retention time of LAB of 2400 s, required for the completion of the reaction. For this end: a) Calculate the volume and dimensions of the mixer tank based on the reaction time given. b) The stirring rate is 100 rpm (rev/min) with what factor should the critical stirring rate be multiple to obtain the actual stirring rate? c) Calculate P, properties d) Calculate the actual power requirement for the two cases given in part (c). e) Calculate the retention time based on the discharge and power numbers. If there is a discrepancy between the retention time required for the completion of reaction and that calculated from power number, give the possible explanations. ) Find the interfacial area for the reaction. fhuld based on 1) Average fluid properties, 2) continuous phase fluid
Step by Step Solution
★★★★★
3.45 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


