Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An uncoated steel (assumed to be iron for simplicity) pipeline is proposed for the transfer of a deaerated weak acidic solution. Define the anodic
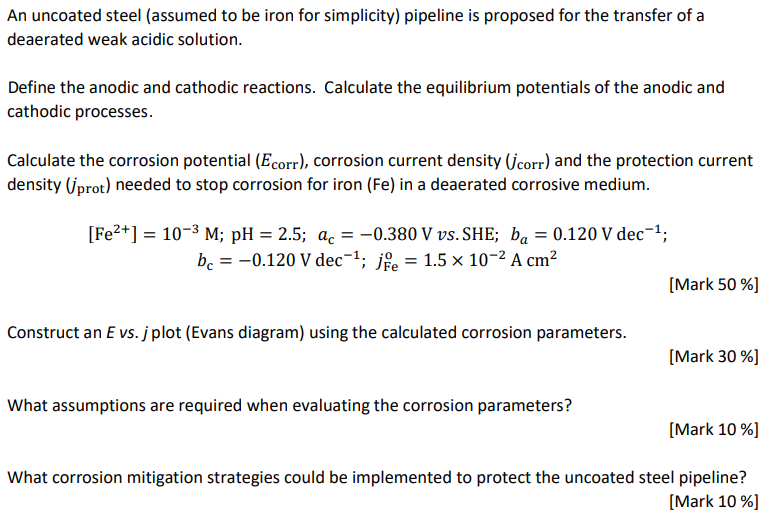
An uncoated steel (assumed to be iron for simplicity) pipeline is proposed for the transfer of a deaerated weak acidic solution. Define the anodic and cathodic reactions. Calculate the equilibrium potentials of the anodic and cathodic processes. Calculate the corrosion potential (Ecorr), corrosion current density (corr) and the protection current density (prot) needed to stop corrosion for iron (Fe) in a deaerated corrosive medium. [Fe2+] = 10-3 M; pH = 2.5; ac = -0.380 V vs. SHE; ba = 0.120 V dec; bc -0.120 V dec; j = 1.5 10- A cm [Mark 50%] Construct an E vs. j plot (Evans diagram) using the calculated corrosion parameters. [Mark 30%] What assumptions are required when evaluating the corrosion parameters? [Mark 10%] What corrosion mitigation strategies could be implemented to protect the uncoated steel pipeline? [Mark 10%]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


