Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An undesired contaminant is present in a fluid. The contaminant is observed to de- grade spontaneously, so a suitable treatment technique is simply to
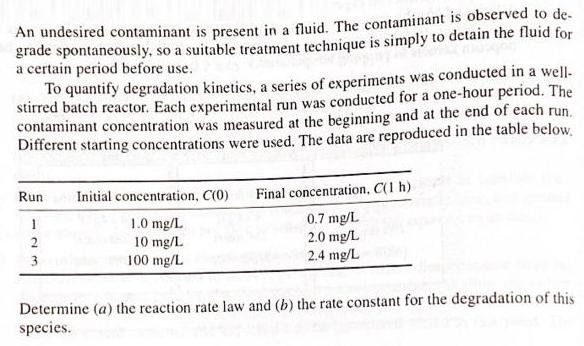
An undesired contaminant is present in a fluid. The contaminant is observed to de- grade spontaneously, so a suitable treatment technique is simply to detain the fluid for a certain period before use. To quantify degradation kinetics, a series of experiments was conducted in a well- stirred batch reactor. Each experimental run was conducted for a one-hour period. The contaminant concentration was measured at the beginning and at the end of each run. Different starting concentrations were used. The data are reproduced in the table below. Run Initial concentration, C(0) Final concentration, C(1 h) 1.0 mg/L 10 mg/L. 100 mg/L 0.7 mg/L 2.0 mg/L 2.4 mg/L 2 3 Determine (a) the reaction rate law and (b) the rate constant for the degradation of this species.
Step by Step Solution
★★★★★
3.46 Rating (172 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


