Question
An unknown protein was determined using the following method: Bradford method Reagents Stock Bradford reagent: Dissolve 100 mg Coomassie Brilliant Blue G250 in a mixture
An unknown protein was determined using the following method:
Bradford method
Reagents
Stock Bradford reagent: Dissolve 100 mg Coomassie Brilliant Blue G250 in a mixture consisting of 100ml of 85% phosphoric acid, 50ml of 95% ethanol and 50ml 1M NaOH. Store at 4°C until precipitation occurs, at which point it is discarded.
Working Bradford reagent: Prepare fresh by diluting 10ml of stock Bradford reagent to 250ml with distilled water.
Stock bovine serum albumin (BSA) solution (10mg/ml): Dissolve 0.2g BSA in 20ml distilled water.
Working BSA concentration range: 0.5 – 1.25 mg/ml
Method
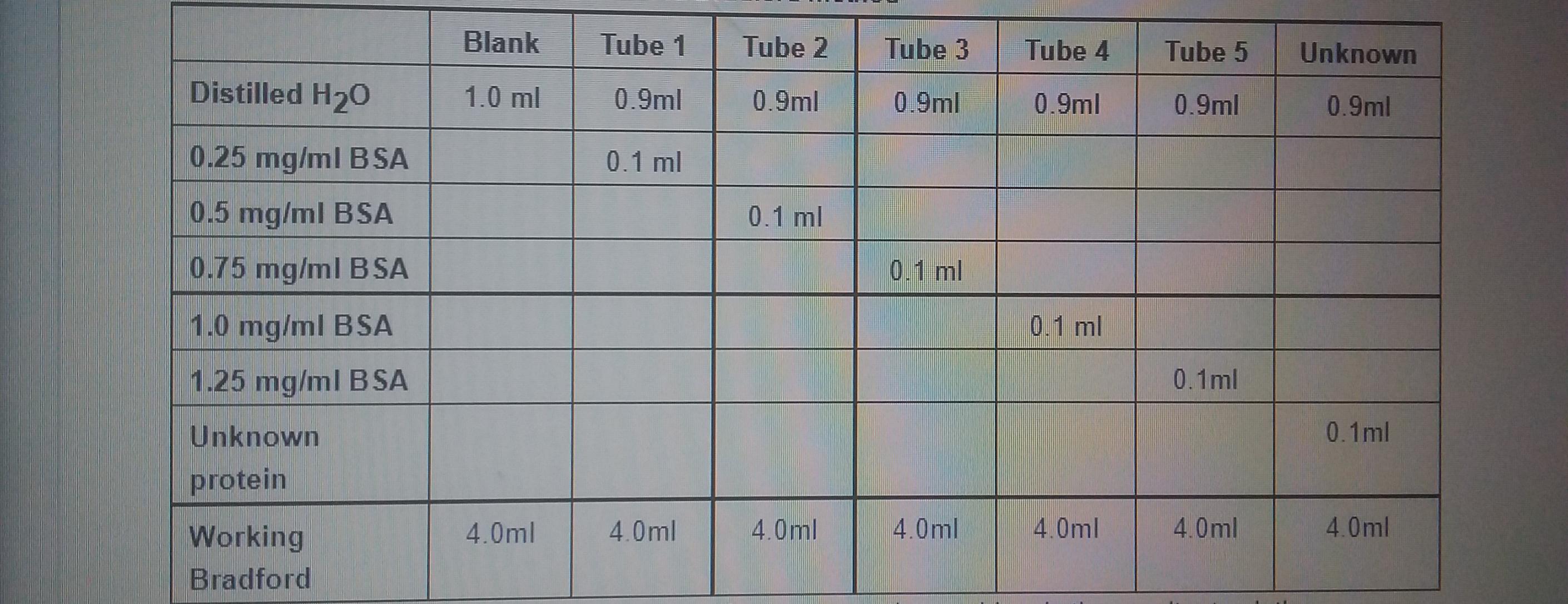
1. Prepare the following test tubes:
Table 1: Preparation of test tubes for the Bradford method
2. After each addition of the working Bradford reagent, thoroughly mix the resultant solution.
3. Incubate the solution at room temperature for 5 min.
4. Read the absorbance at 595nm of the blank and test solutions.
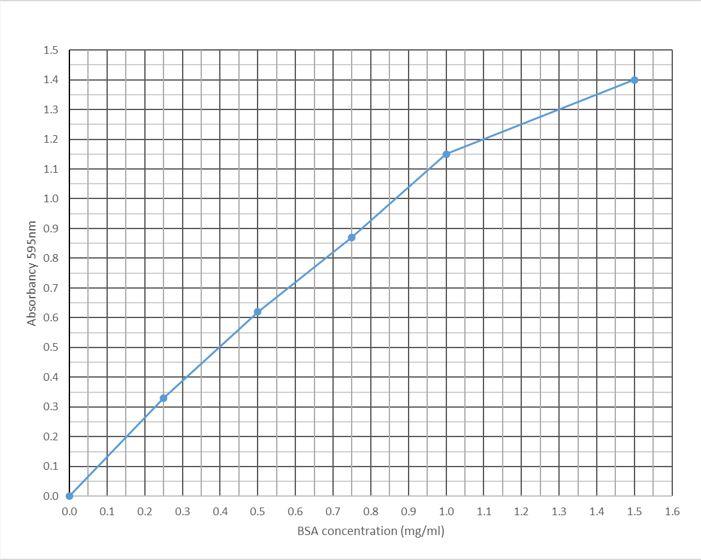
Calculate the protein concentration (in mg/ml) of an unknown sample with an absorbancy value of 0.349 using the standard curve below.
Blank Tube 1 Tube 2 Tube 3 Tube 4 Tube 5 Unknown Distilled H20 1.0 ml 0.9ml 0.9ml 0.9ml 0.9ml 0.9ml 0.9ml 0.25 mg/ml BSA 0.1 ml 0.5 mg/ml BSA 0.1 ml 0.75 mg/ml B SA 0.1 ml 1.0 mg/ml BSA 0.1 ml 1.25 mg/ml B SA 0.1ml Unknown 0.1ml protein Working 4.0ml 4.0ml 4.0ml 4.0ml 4.0ml 4.0ml 4.0ml Bradford
Step by Step Solution
3.52 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
In Ihe given plot o standard curve We havve 05t 04 anb sobance Nalue 0365 1027 0368 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started