Question
An unknown volatile liquid was evaporated in a 250mL container. Use the data below to solve the exercise. (R-8.206102 L atm/K mol) (Theoretical molecular
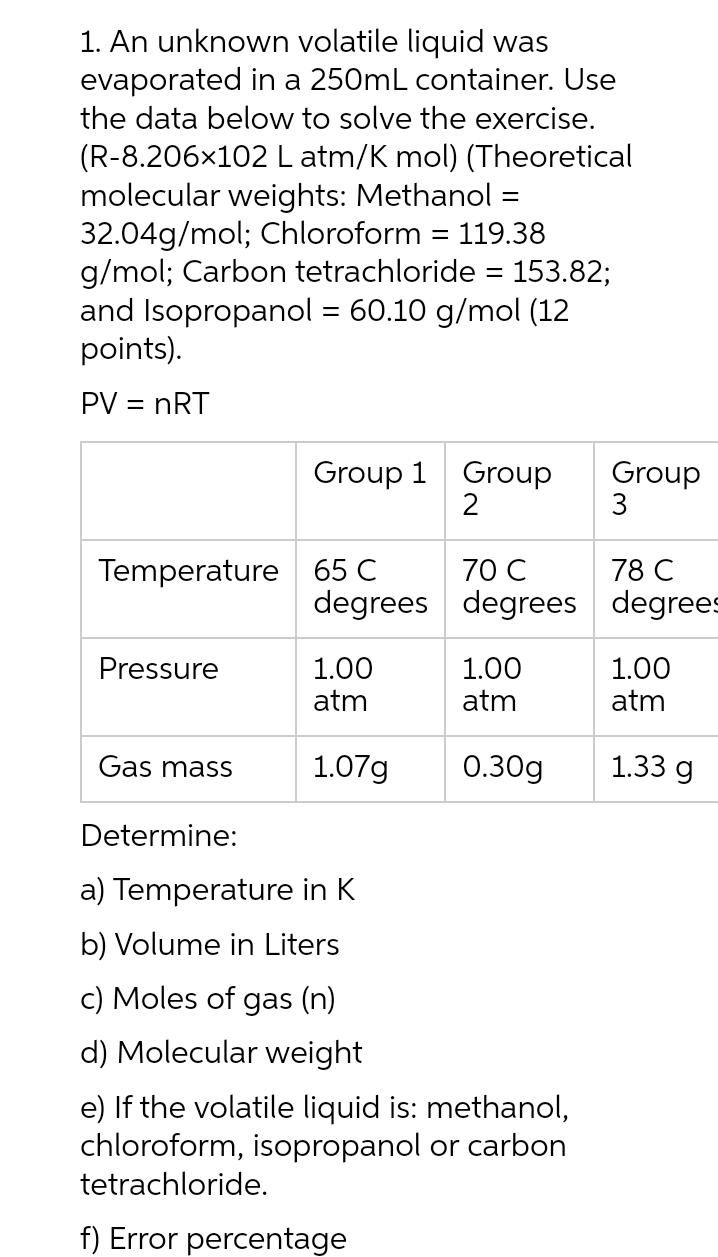
An unknown volatile liquid was evaporated in a 250mL container. Use the data below to solve the exercise. (R-8.206102 L atm/K mol) (Theoretical molecular weights: Methanol = 32.04g/mol; Chloroform = 119.38 g/mol; Carbon tetrachloride = 153.82; and Isopropanol = 60.10 g/mol (12 points). PV = nRT Temperature 65 C Pressure Gas mass Group 1 Group 2 Determine: degrees 1.00 atm 1.07g a) Temperature in K b) Volume in Liters c) Moles of gas (n) d) Molecular weight 70 C degrees 1.00 atm 0.30g e) If the volatile liquid is: methanol, chloroform, isopropanol or carbon tetrachloride. f) Error percentage Group 3 78 C degrees 1.00 atm 1.33 g
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Investments Valuation and Management
Authors: Bradford D. Jordan, Thomas W. Miller
5th edition
978-007728329, 9780073382357, 0077283295, 73382353, 978-0077283292
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



