Answered step by step
Verified Expert Solution
Question
1 Approved Answer
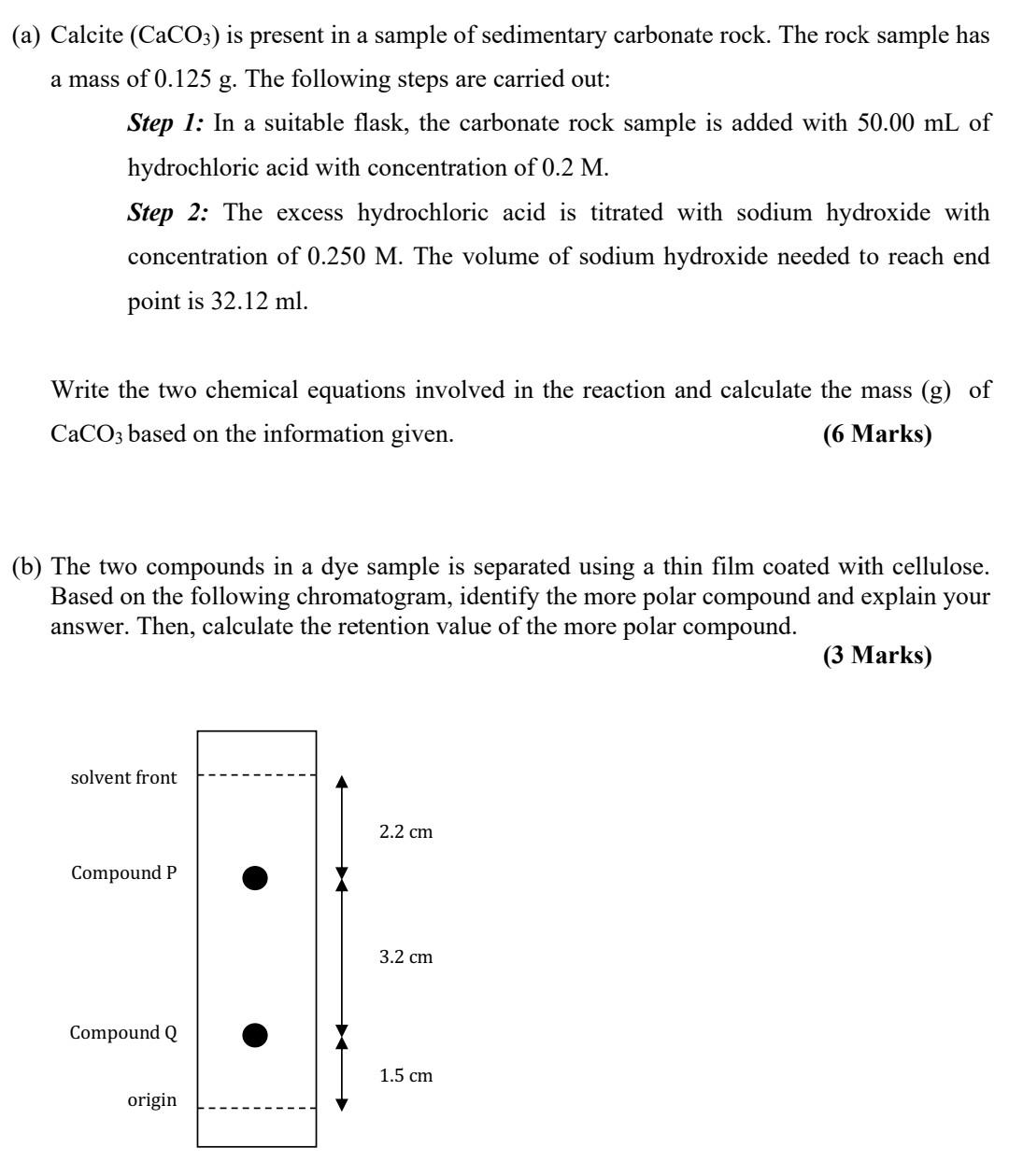
analytical chemistry help pls (a) Calcite (CaCO3) is present in a sample of sedimentary carbonate rock. The rock sample has a mass of 0.125 g.
analytical chemistry help pls
(a) Calcite (CaCO3) is present in a sample of sedimentary carbonate rock. The rock sample has a mass of 0.125 g. The following steps are carried out: Step 1: In a suitable flask, the carbonate rock sample is added with 50.00 mL of hydrochloric acid with concentration of 0.2 M. Step 2: The excess hydrochloric acid is titrated with sodium hydroxide with concentration of 0.250 M. The volume of sodium hydroxide needed to reach end point is 32.12 ml. Write the two chemical equations involved in the reaction and calculate the mass (g) of CaCO3 based on the information given. (6 Marks) (b) The two compounds in a dye sample is separated using a thin film coated with cellulose. Based on the following chromatogram, identify the more polar compound and explain your answer. Then, calculate the retention value of the more polar compound. (3 Marks) solvent front 2.2 cm Compound P 3.2 cm Compound Q 1.5 cm origin ATOMIC Number 3 5 8 9 Li H 4 Be Beryllium 9.012183 Symbol B 6 C Carbon 12.011 7 N Nitrogen 14.007 F 10 Ne Neon 20.180 Boron Lithium 7.0 Hydrogen 1.0080 Name Atomic Mass, u Oxygen 15.999 Fluorine 18.998403 10.81 16 17 18 11 12 Na Sodium Magnesi... 22.989769 24.305 Mg 13 Aluminum 26.981538 14 15 Si P. Silicon Phospho... 28.085 30.9737621 S CI Ar Chlorine Sulfur 32.07 Argon 39.9 35.45 19 27 28 30 31 32 36 K 20 Ca Calcium 40.08 21 22 23 24 25 Sc Ti V Cr Mn Scandium Titanium Vanadium Chromium Mangane... 44.95591 47.867 50.9415 51.996 54.93804 26 Fe Iron 55.84 Co Ni 29 Cu Copper Zn Gallium German... Ga Ge 33 As Arsenic 74.92159 34 Se Selenium 78.97 35 Br Bromine 79.90 Kr Potassium 39.0983 Cobalt 58.93319 Nickel 58.693 Zinc 65.4 Krypton 83.80 63.55 69.723 72.63 47 37 38 Rb Sr Rubidium Strontium 85.468 87.62 39 Y Yttrium 88.90584 40 Zr Zirconium 91.22 41 42 43 44 45 46 Nb Mo Tc Ru Rh Pd Niobium Molybde... Techneti... Ruthenium Rhodium Palladium 92.90637 95.95 96.90636 101.1 102.9055 106.42 Ag 48 Cd Cadmium 112.41 49 50 51 52 In Sn Sb Te Indium Tin Antimony Tellurium 53 I lodine 126.9045 54 Xe Xenon 131.29 Silver 107.868 114.818 118.71 121.760 127.6 55 56 76 78 80 82 Cs Ba 72 Hf Hafnium 178.49 73 74 75 Ta W Tantalum Tungsten Rhenium 180.9479 183.84 186.207 Re Os 77 Ir Iridium 192.22 Pt 79 Au Gold 196.96657 Hg 81 TI Thallium 204.383 Pb 83 84 85 86 Bi At Rn Bismuth Polonium Astatine Radon 208.98040 208.98243 209.98715 222.01758 Po Platinum Cesium 132.90545 Barium 137.33 Osmium 190.2 Mercury 200.59 Lead 207 195.08 87 88 Fr Ra Francium Radium 223.01973 226.02541 Hs ** 108 109 110 111 112 113 114 115 116 117 118 Mt Ds Rg Cn Nh FI Mc Lv Og Hassium Meitneri... Darmsta... Roentge... Copernic... Nihonium Flerovium Moscovi... Livermori... Tennessi... Oganesson 269.1336 277.154 282.166 282.169 286.179 286.182 290.192 290.196 293.205 294.211 295.216 TS 104 105 106 107 Rf Db Sg Bh Rutherfo... Dubnium Seaborgi... Bohrium 267.122 268.126 269.128 270.133 F-Table / df,=1 2 3 4 5 6 7 8 9 10 12 15 20 24 30 40 60 120 8 df_=1 161.4476 199.5000 215.7073 224.5832 230.1619 233.9860 236.7684 238.8827 240.5433 241.8817 243.9060 245.9499 248.0131 249.0518 250.0951 251.1432 252.1957 253.2529 254.3144 2 18.5128 19.0000 19.1643 19.2468 19.2964 19.3295 19.3532 19.3710 19.3848 19.3959 19.4125 19.4291 19.4458 19.4541 19.4624 19.4707 19.4791 19.4874 19.4957 3 10.1280 9.5521 9.2766 9.1172 9.0135 8.9406 8.8867 8.8452 8.8123 8.7855 8.7446 8.7029 8.6602 8.6385 8.6166 8.5944 8.5720 8.5494 8.5264 4 7.7086 6.9443 6.5914 6.3882 6.2561 6.1631 6.0942 6.0410 5.9988 5.9644 5.9117 5.8578 5.8025 5.7744 5.7459 5.7170 5.6877 5.6581 5.6281 5 6.6079 5.7861 5.4095 5.1922 5.0503 4.9503 4.8759 4.8183 4.7725 4.7351 4.6777 4.6188 4.5581 4.5272 4.4957 4.4638 4.4314 4.3985 4.3650 6 5.9874 5.1433 4.7571 4.5337 4.3874 4.2839 4.2067 4.1468 4.0990 4.0600 3.9999 3.9381 3.8742 3.8415 3.8082 3.7743 3.7398 3.7047 3.6689 7 5.5914 4.7374 4.3468 4.1203 3.9715 3.8660 3.7870 3.7257 3.6767 3.6365 3.5747 3.5107 3.4445 3.4105 3.3758 3.3404 3.3043 3.2674 3.2298 8 5.3177 4.4590 4.0662 3.8379 3.6875 3.5806 3.5005 3.4381 3.3881 3.3472 3.2839 3.2184 3.1503 3.1152 3.0794 3.0428 3.0053 2.9669 2.9276 9 5.1174 4.2565 3.8625 3.6331 3.4817 3.3738 3.2927 3.2296 3.1789 3.1373 3.0729 3.0061 2.9365 2.9005 2.8637 2.8259 2.7872 2.7475 2.7067 10 4.9646 4.1028 3.7083 3.4780 3.3258 3.2172 3.1355 3.0717 3.0204 2.9782 2.9130 2.8450 2.7740 2.7372 2.6996 2.6609 2.6211 2.5801 2.5379 11 4.8443 3.9823 3.5874 3.3567 3.2039 3.0946 3.0123 2.9480 2.8962 2.8536 2.7876 2.7186 2.6464 2.6090 2.5705 2.5309 2.4901 2.4480 2.4045 12 4.7472 3.8853 3.4903 3.2592 3.1059 2.9961 2.9134 2.8486 2.7964 2.7534 2.6866 2.6169 2.5436 2.5055 2.4663 2.4259 2.3842 2.3410 2.2962 13 4.6672 3.8056 3.4105 3.1791 3.0254 2.9153 2.8321 2.7669 2.7144 2.6710 2.6037 2.5331 2.4589 2.4202 2.3803 2.3392 2.2966 2.2524 2.2064 14 4.6001 3.7389 3.3439 3.1122 2.9582 2.8477 2.7642 2.6987 2.6458 2.6022 2.5342 2.4630 2.3879 2.3487 2.3082 2.2664 2.2229 2.1778 2.1307 15 4.5431 3.6823 3.2874 3.0556 2.9013 2.7905 2.7066 2.6408 2.5876 2.5437 2.4753 2.4034 2.3275 2.2878 2.2468 2.2043 2.1601 2.1141 2.0658Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


