Answered step by step
Verified Expert Solution
Question
1 Approved Answer
and rate constants for two parallel, irreversible reactions. We consider two situations: (1) parallel first-order decomposition (single reactant) reactions, and (2) competitive parallel reactions with
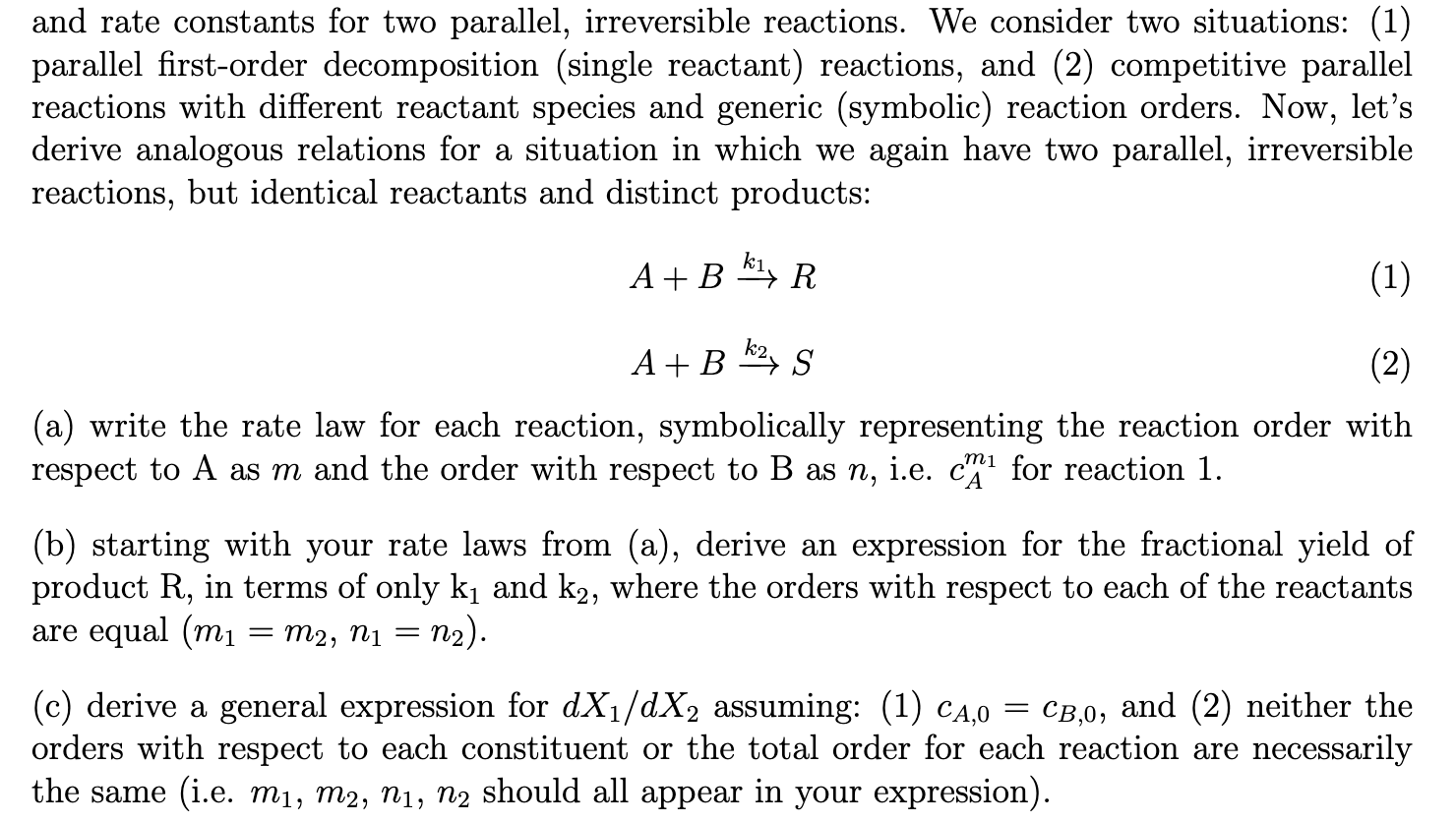 and rate constants for two parallel, irreversible reactions. We consider two situations: (1) parallel first-order decomposition (single reactant) reactions, and (2) competitive parallel reactions with different reactant species and generic (symbolic) reaction orders. Now, let's derive analogous relations for a situation in which we again have two parallel, irreversible reactions, but identical reactants and distinct products: A+Bk1RA+Bk2S (a) write the rate law for each reaction, symbolically representing the reaction order with respect to A as m and the order with respect to B as n, i.e. cAm1 for reaction 1 . (b) starting with your rate laws from (a), derive an expression for the fractional yield of product R, in terms of only k1 and k2, where the orders with respect to each of the reactants are equal (m1=m2,n1=n2) (c) derive a general expression for dX1/dX2 assuming: (1) cA,0=cB,0, and (2) neither the orders with respect to each constituent or the total order for each reaction are necessarily the same (i.e. m1,m2,n1,n2 should all appear in your expression)
and rate constants for two parallel, irreversible reactions. We consider two situations: (1) parallel first-order decomposition (single reactant) reactions, and (2) competitive parallel reactions with different reactant species and generic (symbolic) reaction orders. Now, let's derive analogous relations for a situation in which we again have two parallel, irreversible reactions, but identical reactants and distinct products: A+Bk1RA+Bk2S (a) write the rate law for each reaction, symbolically representing the reaction order with respect to A as m and the order with respect to B as n, i.e. cAm1 for reaction 1 . (b) starting with your rate laws from (a), derive an expression for the fractional yield of product R, in terms of only k1 and k2, where the orders with respect to each of the reactants are equal (m1=m2,n1=n2) (c) derive a general expression for dX1/dX2 assuming: (1) cA,0=cB,0, and (2) neither the orders with respect to each constituent or the total order for each reaction are necessarily the same (i.e. m1,m2,n1,n2 should all appear in your expression) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


