Question
Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or
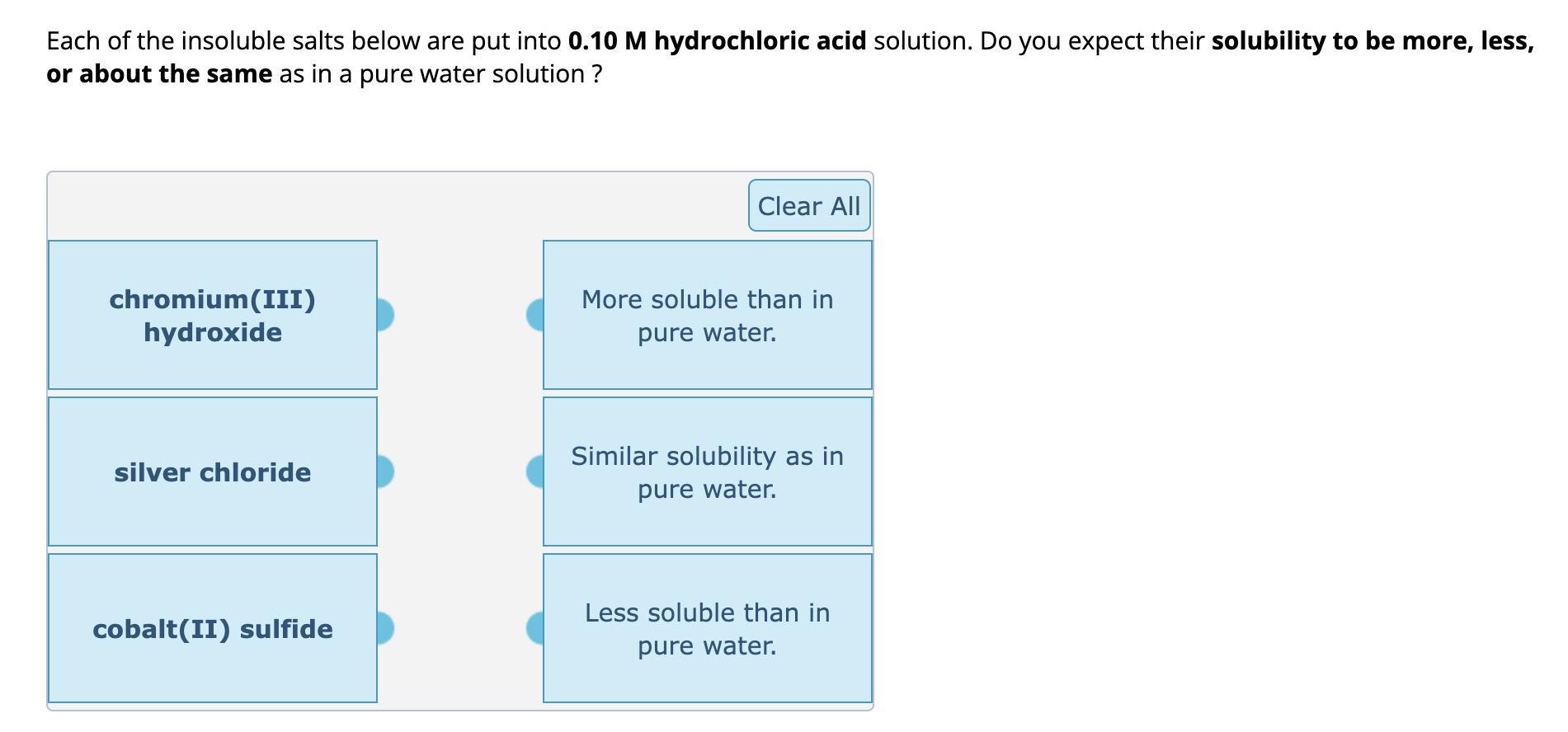
Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution? chromium(III) hydroxide Clear All More soluble than in pure water. silver chloride Similar solubility as in pure water. cobalt(II) sulfide Less soluble than in pure water.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
International Financial Reporting and Analysis
Authors: David Alexander, Anne Britton, Ann Jorissen
5th edition
978-1408032282, 1408032287, 978-1408075012
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



