answer a-d
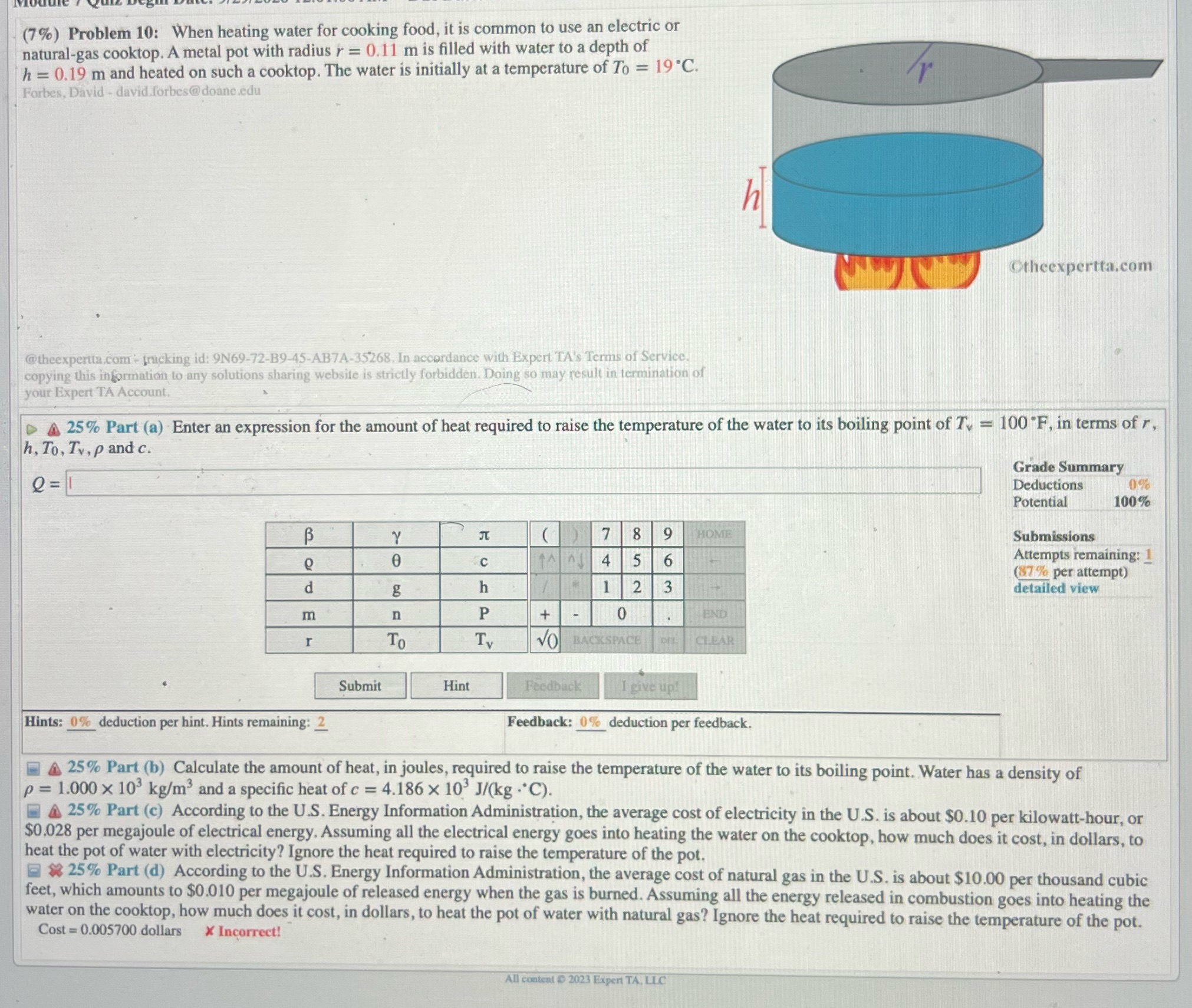
(7%) Problem 10: When heating water for cooking food, it is common to use an electric or natural-gas cooktop. A metal pot with radius r = 0.11 m is filled with water to a depth of h = 0.19 m and heated on such a cooktop. The water is initially at a temperature of To = 19.C. r Forbes, David - david.forbes@doane.edu Otheexpertta.com @theexpertta.com + fracking id: 9N69-72-B9-45-AB7A-35268. In accordance with Expert TA's Terms of Service. copying this information to any solutions sharing website is strictly forbidden. Doing so may result in termination of your Expert TA Account. 4 25% Part (a) Enter an expression for the amount of heat required to raise the temperature of the water to its boiling point of Tv = 100 F, in terms of r, h, To, Tv, p and c. Grade Summary e=1 Deductions 0% Potential 100% B Y ( ) 7 8 9 HOME Submissions 0 C 6 Attempts remaining: 1 (87 % per attempt) d g h 1 2 3 detailed view m n P + - 0 END To Ty VO CLEAR Submit Hint Feedback I give up! Hints: 0% deduction per hint. Hints remaining: 2 Feedback: 0% deduction per feedback. 4 25% Part (b) Calculate the amount of heat, in joules, required to raise the temperature of the water to its boiling point. Water has a density of p = 1.000 X 10' kg/m' and a specific heat of c = 4.186 x 10' J/(kg . C). A 25% Part (c) According to the U.S. Energy Information Administration, the average cost of electricity in the U.S. is about $0.10 per kilowatt-hour, or $0.028 per megajoule of electrical energy. Assuming all the electrical energy goes into heating the water on the cooktop, how much does it cost, in dollars, to heat the pot of water with electricity? Ignore the heat required to raise the temperature of the pot. * 25% Part (d) According to the U.S. Energy Information Administration, the average cost of natural gas in the U.S. is about $10.00 per thousand cubic feet, which amounts to $0.010 per megajoule of released energy when the gas is burned. Assuming all the energy released in combustion goes into heating the water on the cooktop, how much does it cost, in dollars, to heat the pot of water with natural gas? Ignore the heat required to raise the temperature of the pot. Cost = 0.005700 dollars X Incorrect! All content @ 2023 Expert TA, LLC