Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) A portion of dilute mixed base solution (25 cm), containing sodium carbonate and sodium hydrogen carbonate was titrated potentiometrically against standardised HO (0.1623
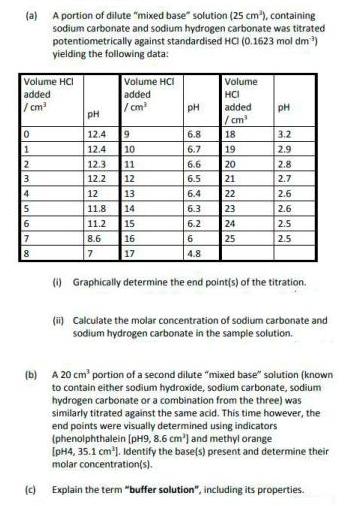
(a) A portion of dilute "mixed base" solution (25 cm), containing sodium carbonate and sodium hydrogen carbonate was titrated potentiometrically against standardised HO (0.1623 mol dm) yielding the following data: Volume HCI added / cm Volume HCI added /cm Volume HCI added / cm 18 19 pH pH pH 12.4 19 6.8 3.2 12.4 10 11 6.7 2.9 12.3 6.6 20 2.8 21 22 23 24 25 3 12.2 12 6.5 2.7 4 12 13 6.4 2.6 11.8 14 6.3 2.6 15 16 17 6 11.2 6.2 2.5 7 8.6 6 2.5 8 7 4.8 (i) Graphically determine the end point(s) of the titration. (i) Calculate the molar concentration of sodium carbonate and sodium hydrogen carbonate in the sample solution. (b) A 20 cm' portion of a second dilute "mixed base" solution (known to contain either sodium hydroxide, sodium carbonate, sodium hydrogen carbonate or a combination from the three) was similarly titrated against the same acid. This time however, the end points were visually determined using indicators (phenolphthalein [pH9, 8.6 cm') and methyl orange [pH4, 35.1 cm). Identify the base(s) present and determine their molar concentration(s). (c) Explain the term "buffer solution", including its properties.
Step by Step Solution
★★★★★
3.41 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
From the graph with xaxis representing volume of acid and Yaxis representing pH values First inflect...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


