Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer all parts and label clearly The information below can be used to help answer the 6qs above Question 1. Electrical conduction is a property
answer all parts and label clearly 


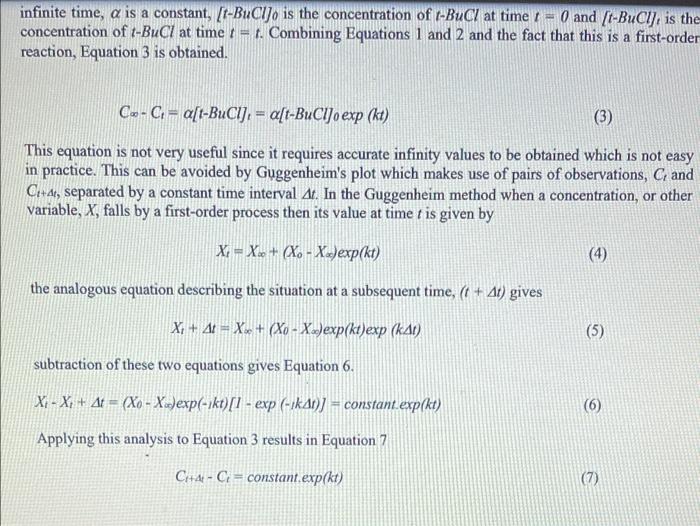
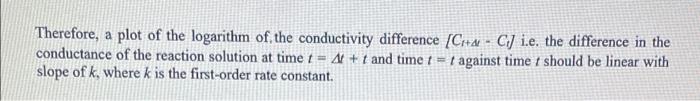
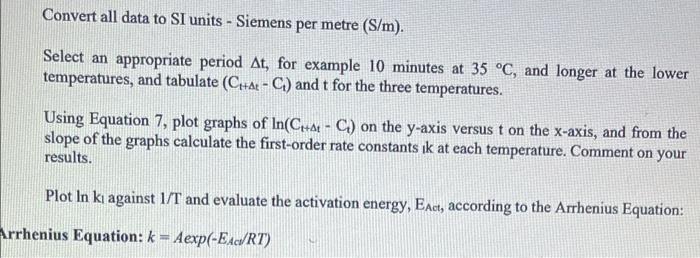
Question 1. Electrical conduction is a property of ionic solutions and the conductance of such electrolytic solutions depends on the concentration of the ions and also the nature of the ions (charges and mobilities). Given that electrolyte solutions obey Ohms law-express the resistance Rof a solution in ohms (). - A conductivity cell was calibrated using 0.01 M KCl (K = 0.1408 S m' at 25C) and the measured resistance was 688 m... Find the cell constant (UA) (m) 1) What are the two factors that determine the conductivity of a solution? 2) Why are temperatures of 35C, 25C and Room Temperature required? 3) What is Enc? 4) Is k the same for all temperatures? Give a brief explanation to your answer. a 5) If you performed this reaction at 50 C, would you expect the k value to be higher or lower than that obtained for the reaction performed at 35C? Explain your reasoning for you answer. 6) If this chemical reaction followed 2nd order kinetics, how you you graph your data to obtain the k values for each temperature. Objective: The objective of this experiment is to monitor the reaction between t-butylchloride and an aqueous alcohol at various temperatures by monitoring the decrease in reactant concentration via the conductivity of the solutions. To then determine the rate constants, k, for this 1" order reaction through the integrated rate law by graphing time against Ln (conc) for the various temperatures. And to finally use the individual k values for each temperature and the Arrhenius Equation and determine the activation energy Es, for the chemical reaction. Introduction In principle, any reaction involving a change in the number or nature of ions can be followed by conductivity measurements. Since these measurements can be recorded easily and can be very sensitive, they are especially appropriate for fast reactions or those involving very low ionic concentrations. The system studied in the present experiment, t-butyl chloride hydrolysed in aqueous alcohol, is a first- order reaction producing HCl as a product of the reaction. The conductance measured is proportional to the HCl concentration, which is equal to the concentration of t-BuCl consumed. Hence C = Co + a[[t-BuClo - [t-BUCI:} and (1) Co = Co + a[t-BuCI]. (2) where C, is the conductance at time t = 4, Co is the conductance at time r = 0, C, is the conductance at infinite time, a is a constant, [t-Bucijo is the concentration of t-BuCl at time r = 0 and (t-BuCI, is the concentration of t-BuCl at time != t. Combining Equations 1 and 2 and the fact that this is a first-order reaction, Equation 3 is obtained. C.-C. = a[t-BuCIJ. = a[t-BuClJo exp (kt) (3) infinite time, a is a constant, [t-BuCIJo is the concentration of t-BuCl at time - O and [t-BuCl], is the concentration of t-BuCl at time = t. Combining Equations 1 and 2 and the fact that this is a first-order reaction, Equation 3 is obtained. Co-C = a[t-BuCI), = a[t-BuCIJo exp (kt) (3) This equation is not very useful since it requires accurate infinity values to be obtained which is not easy in practice. This can be avoided by Guggenheim's plot which makes use of pairs of observations, C, and Cl+at, separated by a constant time interval At. In the Guggenheim method when a concentration, or other variable, X, falls by a first-order process then its value at time t is given by X; - X. + (X. - X.)exp(kt) the analogous equation describing the situation at a subsequent time, (1 + At) gives X + 4 = X + (Xo - Xexp(kt exp (kat) (5) subtraction of these two equations gives Equation 6. XI - X + At = (Xo - Xo)exp(- kt) [1 - exp( -AN) constant.exp(kt) (6) Applying this analysis to Equation 3 results in Equation 7 CH+4- Ci= constant exp(kt) (7) Therefore, a plot of the logarithm of the conductivity difference (Crta - CJ i.e. the difference in the conductance of the reaction solution at time t = 4 + t and time 1 - 1 against time I should be linear with slope of k, where k is the first-order rate constant. Convert all data to SI units - Siemens per metre (S/m). Select an appropriate period At, for example 10 minutes at 35 C, and longer at the lower temperatures, and tabulate (Chae - C) and t for the three temperatures. Using Equation 7, plot graphs of In(Citd - C) on the y-axis versus t on the x-axis, and from the slope of the graphs calculate the first-order rate constants ik at each temperature. Comment on your results. Plot In k against 1/T and evaluate the activation energy, East, according to the Arrhenius Equation: Arrhenius Equation: k = Aexp(-EART) 

The information below can be used to help answer the 6qs above

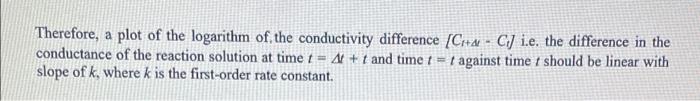

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


