Answered step by step
Verified Expert Solution
Question
1 Approved Answer
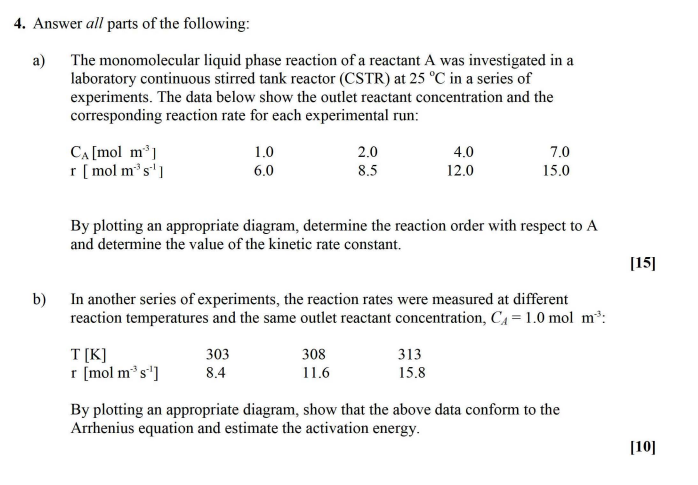
Answer all parts of the following: a ) The monomolecular liquid phase reaction of a reactant A was investigated in a laboratory continuous stirred tank
Answer all parts of the following:
a The monomolecular liquid phase reaction of a reactant A was investigated in a
laboratory continuous stirred tank reactor CSTR at in a series of
experiments. The data below show the outlet reactant concentration and the
corresponding reaction rate for each experimental run:
By plotting an appropriate diagram, determine the reaction order with respect to A
and determine the value of the kinetic rate constant.
b In another series of experiments, the reaction rates were measured at different
reaction temperatures and the same outlet reactant concentration, :
By plotting an appropriate diagram, show that the above data conform to the
Arrhenius equation and estimate the activation energy.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


