Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer and code please The temperature dependence of chemical reactions can be computed with the Arrhenius equation: k=AeEN(RTe) where k= reaction rate (s1),A= frequency (s1),E=
answer and code please 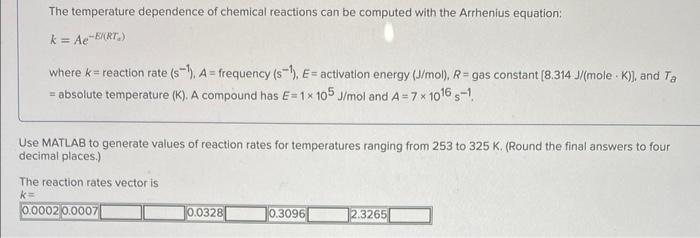
The temperature dependence of chemical reactions can be computed with the Arrhenius equation: k=AeEN(RTe) where k= reaction rate (s1),A= frequency (s1),E= activation energy (J/mol),R= gas constant [8.314J/(moleK), and Ta = absolute temperature (K). A compound has E=1105J/mol and A=71016s1. Use MATLAB to generate values of reaction rates for temperatures ranging from 253 to 325K. (Round the final answers to four decimal places.) The reaction rates vector is k= 0.00020.0007 0.0328 0.3096 2.3265 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


