Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer Part E and the Critical thinking ans communication questions please Density Lab Part 2 Based on Work from Lab Simulations by Hayden-McNeil.
Answer Part E and the " Critical thinking ans communication " questions please 
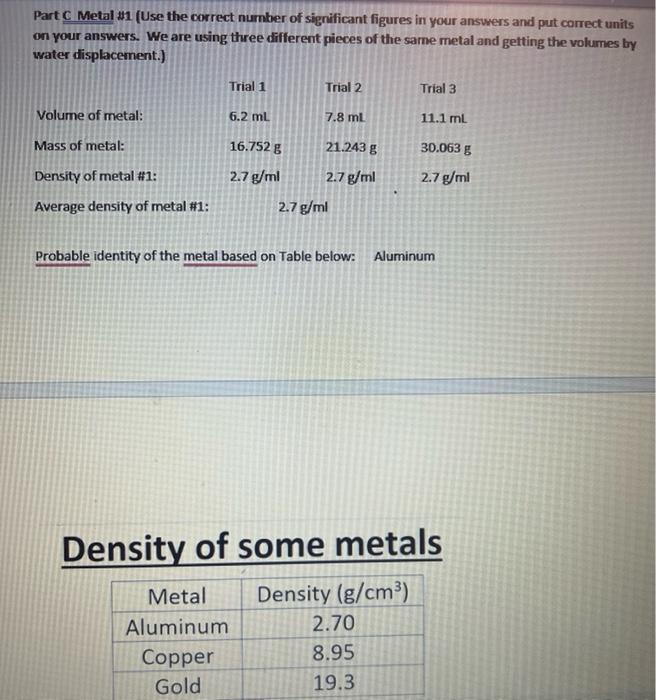
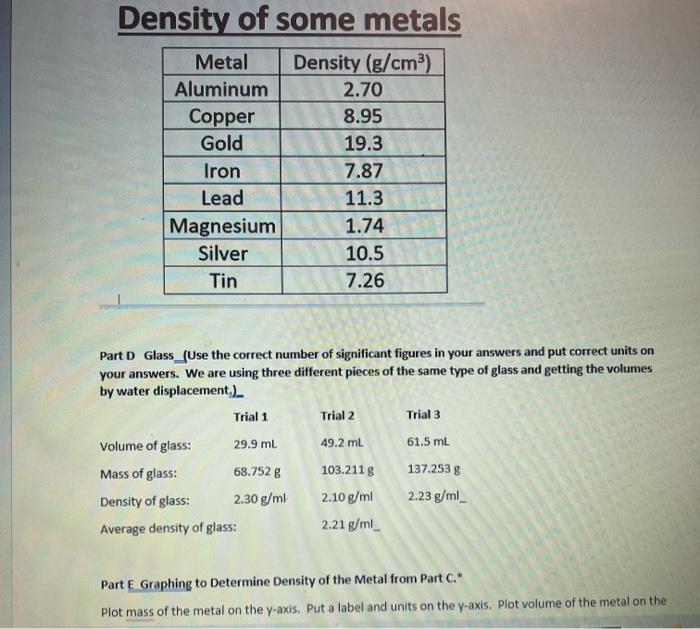
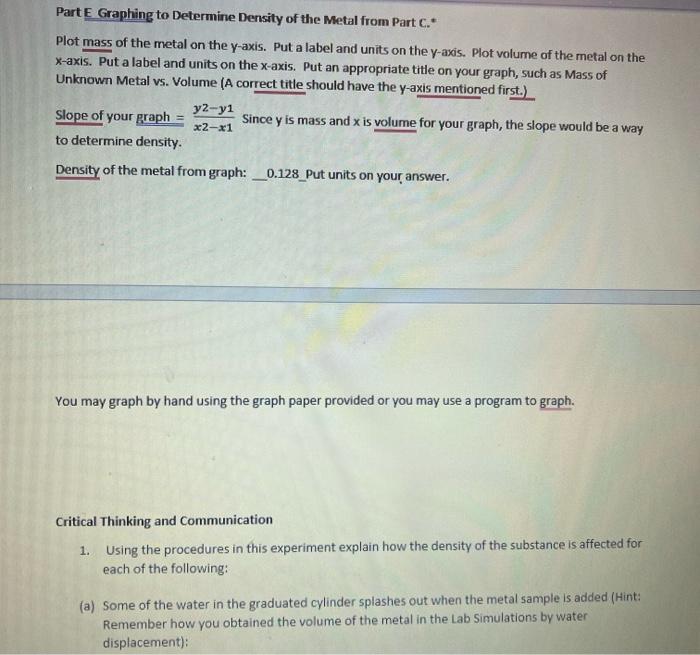

Density Lab Part 2 Based on Work from Lab Simulations by Hayden-McNeil. Critical Thinking and Communication After using the Hayden-McNeil Lab Simulations and generating your own data for two liquids and two solids to determine the density of each, use the following data to determine densities. Part A Water (Use the correct number of significant figures in your answers and put correct units on your answers.) Trial 1 Trial 2 Trial 3 10.0 mL 10.0 mL 10.0 ml Volume of water: Mass of water: Density of water: Average density of water: 9.988 g 9.995 g 9.990 g _0.9988 g/ml 0.9995 g/ml _0.9991 g/ml _0.9990 g/ml_ Part B Salt Solution (Use the correct number of significant figures in your answers and put correct units on your answers.) Trial 1 Trial 2 Trial 3 Volume of salt solution: 10.0 mL 10.0 ml 10.0 mL Mass of salt solution: 10.665 g 10.762 g 10.567 g Density of salt solution: 1.0665 g/ml 1.0762 g/ml 1.0567 g/ml Average density of salt solution: 1.0664 g/ml Part C Metal #1 (Use the correct number of significant figures in your answers and put correct units in our ancrore La are nicing three different nierac of the cama motal and notting the valumac hw Part C Metal 21 (Use the correct number of significant figures in your answers and put correct units on your answers. We are using three different pieces of the same metal and getting the volumes by water displacement.) Trial 1 Trial 2 Trial 3 Volume of metal: 6.2 ml 7.8 ml 11.1 ml Mass of metal: 16.752 g 21.243 g 30.0638 Density of metal #1: 2.7 g/ml 2.7 g/ml 2.7 g/ml 2.7 g/ml Average density of metal #1: Probable identity of the metal based on Table below: Aluminum Density of some metals Metal Density (g/cm3) Aluminum 2.70 Copper 8.95 Gold 19.3 Density of some metals Metal Density (g/cm) Aluminum 2.70 Copper 8.95 Gold 19.3 Iron 7.87 Lead 11.3 Magnesium 1.74 Silver 10.5 Tin 7.26 Part D Glass_(Use the correct number of significant figures in your answers and put correct units on your answers. We are using three different pieces of the same type of glass and getting the volumes by water displacement.) Trial 1 Trial 2 Trial 3 Volume of glass: 29.9 ml 49.2 ml 61.5 mL 137.253 g Mass of glass: 68.752 g Density of glass: 2.30 g/ml Average density of glass: 103.2118 2.10 g/ml 2.21 g/ml_ 2.23 g/ml_ Part E Graphing to Determine Density of the Metal from Part C. Plot mass of the metal on the y-axis. Put a label and units on the y-axis. Plot volume of the metal on the Part E Graphing to Determine Density of the Metal from Part C. Plot mass of the metal on the y-axis. Put a label and units on the y-axis. Plot volume of the metal on the x-axis. Put a label and units on the x-axis. Put an appropriate title on your graph, such as Mass of Unknown Metal vs. Volume (A correct title should have the y-axis mentioned first.) y2-y1 Slope of your graph Since y is mass and x is volume for your graph, the slope would be a way x2-1 to determine density. Density of the metal from graph: _0.128_Put units on your answer. You may graph by hand using the graph paper provided or you may use a program to graph. Critical Thinking and Communication 1. Using the procedures in this experiment explain how the density of the substance is affected for each of the following: (a) Some of the water in the graduated cylinder splashes out when the metal sample is added (Hint: Remember how you obtained the volume of the metal in the Lab Simulations by water displacement): You may graph by hand using the graph paper provided or you may use a program to graph. Critical Thinking and Communication 1. Using the procedures in this experiment explain how the density of the substance is affected for each of the following: (a) Some of the water in the graduated cylinder splashes out when the metal sample is added (Hint: Remember how you obtained the volume of the metal in the Lab Simulations by water displacement): (b) Air bubbles adhere to the submerged metal when the volume of sample plus water is measured: 2. Describe sources of error that may affect your results in a real lab. 3. Explain what would happen to the density of the sample of the sample is cut in half. 22 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


