Answered step by step
Verified Expert Solution
Question
1 Approved Answer
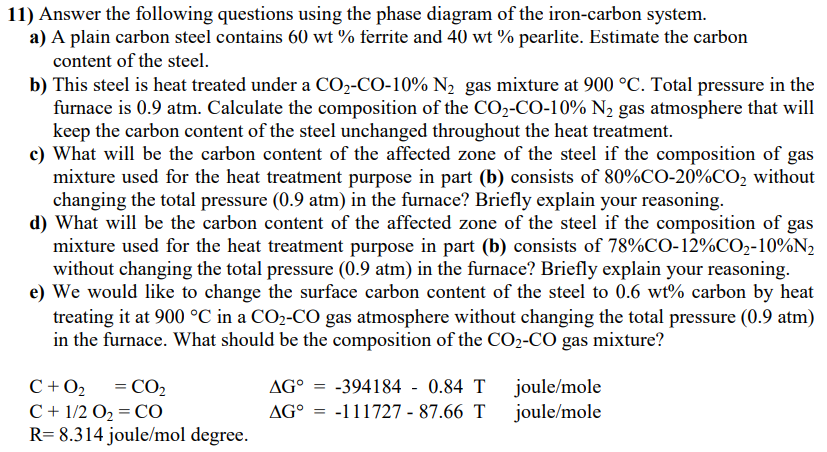
Answer the following questions using the phase diagram of the iron - carbon system. a ) A plain carbon steel contains 6 0 wt %
Answer the following questions using the phase diagram of the ironcarbon system.
a A plain carbon steel contains wt ferrite and wt pearlite. Estimate the carbon
content of the steel.
b This steel is heat treated under a COCO N gas mixture at C Total pressure in the
furnace is atm. Calculate the composition of the COCO N gas atmosphere that will
keep the carbon content of the steel unchanged throughout the heat treatment.
c What will be the carbon content of the affected zone of the steel if the composition of gas
mixture used for the heat treatment purpose in part b consists of COCO without
changing the total pressure atm in the furnace? Briefly explain your reasoning.
d What will be the carbon content of the affected zone of the steel if the composition of gas
mixture used for the heat treatment purpose in part b consists of COCON
without changing the total pressure atm in the furnace? Briefly explain your reasoning.
e We would like to change the surface carbon content of the steel to wt carbon by heat
treating it at C in a COCO gas atmosphere without changing the total pressure atm
in the furnace. What should be the composition of the COCO gas mixture?
C O COG T joulemole
C O CO G T joulemole
R joulemol degree.Answer the following questions using the phase diagram of the ironcarbon system.
a A plain carbon steel contains ferrite and pearlite. Estimate the carbon
content of the steel.
b This steel is heat treated under a gas mixture at Total pressure in the
furnace is atm. Calculate the composition of the gas atmosphere that will
keep the carbon content of the steel unchanged throughout the heat treatment.
c What will be the carbon content of the affected zone of the steel if the composition of gas
mixture used for the heat treatment purpose in part b consists of without
changing the total pressure atm in the furnace? Briefly explain your reasoning.
d What will be the carbon content of the affected zone of the steel if the composition of gas
mixture used for the heat treatment purpose in part b consists of
without changing the total pressure atm in the furnace? Briefly explain your reasoning.
e We would like to change the surface carbon content of the steel to carbon by heat
treating it at in a gas atmosphere without changing the total pressure atm
in the furnace. What should be the composition of the gas mixture?
joule
joule
joule degree.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


